Case Files Biochemistry, 3rd Edition (2015)
SECTION II. Clinical Cases
CASE 41
A 37-year-old woman presents to your clinic to discuss her plans for a new vegetarian diet. The patient heard from a friend about a new vegetarian diet that promised rapid weight loss. The diet consists of many leafy vegetables with no pork, chicken, beef, eggs, or milk. She is also planning on working out regularly with the goal of running a marathon within the year. After listening to the patient, you refer her to a nutritionist for further assistance and guidance.
![]() What is an essential amino acid, and how many are there?
What is an essential amino acid, and how many are there?
![]() List the essential amino acids.
List the essential amino acids.
ANSWERS TO CASE 41:
Vegetarian Diet (Essential Amino Acids)
Summary: A 37-year-old woman who is planning to undertake a radical new vegetarian diet is in your office for counseling.
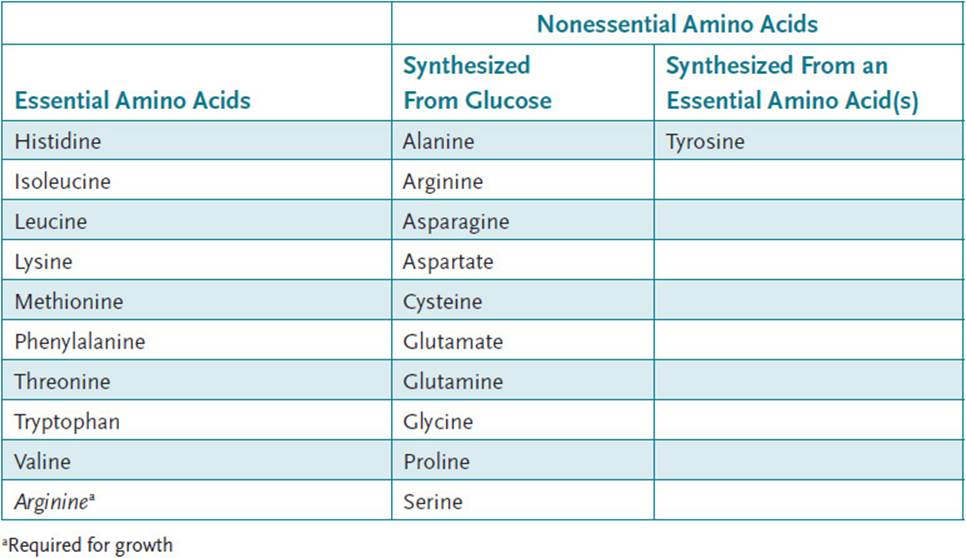
• Essential amino acids: The amino acids that cannot be synthesized by the body. There are a total of nine essential amino acids.
• List of essential amino acids: Histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine.
CLINICAL CORRELATION
The vegetarian should be careful to ensure a balanced ingestion of proteins, fats, carbohydrates, and vitamins. Most animal proteins contain all the essential amino acids; however, vegetable proteins often lack one or more of them. Often, plant amino acids are of low biologic value and incompletely digested. Vegans often benefit from nutritional consultation, which would allow the patient to make sure that she ate foods that complemented each other in providing the essential amino acids.
APPROACH TO:
Essential Amino Acids
OBJECTIVES
1. Explain the importance of essential amino acids.
2. Outline the synthesis of other amino acids.
3. Describe some of the problems that arise with inadequate essential amino acid intake.
DEFINITIONS
ESSENTIAL AMINO ACIDS: Amino acids that the human body cannot synthesize (or cannot synthesize in sufficient quantities to meet cellular needs) and must be taken in the diet. If essential amino acids are deficient, the result is a condition of negative nitrogen balance.
NONESSENTIAL AMINO ACIDS: Those amino acids that are synthesized by the human body in sufficient quantities to meet cellular needs.
PHENYLALANINE HYDROXYLASE: The enzyme that converts the essential amino acid phenylalanine to the amino acid tyrosine using tetrahydrobiopterin and molecular oxygen. A genetic deficiency in this enzyme gives rise to the disease phenylketonuria.
PHENYLKETONES: Normally minor metabolites of phenylalanine that result from the transamination of phenylalanine and further reduction. These include phenylpyruvate and phenylacetate. These metabolites are elevated when the conversion of phenylalanine to tyrosine is impaired.
PKU: Phenylketonuria (PKU); the pathologic condition of increased excretion of phenylketones in the urine because of impaired conversion of phenylalanine to tyrosine. Classical PKU is because of a genetic deficiency of phenylalanine hydroxylase; however, other causes are deficiencies in dihydropteridine reductase or in the biosynthesis of tetrahydrobiopterin.
DISCUSSION
All 20 amino acids are needed for normal cellular growth and function. Amino acids are the basic building blocks for all proteins synthesized in the cell. In addition, the metabolism of amino acids provides carbon and nitrogen units used in the synthesis of numerous important biomolecules including neurotransmitters, heme, purines, pyrimidines, polyamines, and various cellular-signaling molecules. The carbon skeletons of amino acids can also be used as an energy source. After removal of the amino group, amino acids can either be directly oxidized or converted to glucose in the liver, providing carbon units to other tissues for the production of adenosine triphosphate (ATP) through glycolysis and the tricarboxylic acid (TCA) cycle. Certain amino acids can be directly interconverted to intermediates of the TCA cycle to provide a rapid source of carbon units. Finally, catabolism of amino acids provides a means of nitrogen and carbon removal from the body through their metabolism to urea and CO2. Thus, all amino acids are necessary for life.
Apart from their biologic importance, amino acids are classified as essential or nonessential based on their ability to be synthesized in the body. The term essential amino acid is used to identify those amino acids that must be taken in through the diet (Table 41-1). There are 10 such amino acids for which biosynthetic pathways do not exist in cells of the human body. By contrast, there are 11 amino acids that are termed nonessential, for which the human body has biosynthetic pathways for their generation. One of these amino acids, tyrosine, is synthesized from the essential amino acid phenylalanine (Figure 38-2). In addition, it should be noted that arginine is listed as both an essential and nonessential amino acid. Arginine is considered nonessential because biosynthetic pathways for its generation do exist in certain cells of the body. Arginine can be synthesized from the amino acid glutamate. Glutamate is first converted to ornithine, which is then converted to arginine by enzymes of the urea cycle. The urea cycle is found only in the liver, and thus the production of arginine through this pathway is limited. The production of arginine through this pathway is likely sufficient for healthy adults but may not be sufficient in times of growth when increased protein synthesis augments the need for amino acids. Thus, in growing children and in adults following surgery or trauma, arginine becomes an essential amino acid.
Table 41-1 • ESSENTIAL AND NONESSENTIAL AMINO ACIDS
There is a continuous need for amino acids for protein synthesis, energy utilization, and the production of biologic mediators. The most readily available source of all amino acids, but particularly the essential amino acids, is the diet. Protein is taken in through the diet and digested to smaller peptides and amino acids in the stomach and small intestine by specific proteolytic enzymes known as proteases. Because of their different specificities, enzymes work to cleave specific peptide bonds within proteins. Individual digestive enzymes are not capable of completely digesting proteins themselves, but in concert with many different enzymes, most proteins can be efficiently digested. Once released by the digestive enzymes, amino acids are absorbed by epithelial cells of the small intestine for distribution and utilization throughout the body.
Composition of the diet is an important consideration when trying to understand and plan for the uptake of essential amino acids. Not all dietary constituents are equal with regard to the type and amounts of proteins present or the amino acids that can be derived from these proteins by the human digestive tract. Proteins derived from vegetable matter may not contain all the essential amino acids needed and digestion of certain plant proteins can be insufficient to produce certain individual amino acids. By contrast, proteins found in animal products are readily digestible and contain all essential amino acids. Therefore, careful consideration should be given to the dietary intake of individuals who may have or will be undergoing an increased level of exertion.
Regulation of the dietary intake of amino acids can also be important when considering the treatment of certain defects in amino acid biosynthesis. Phenylalanine is an essential amino acid that is also used to generate the nonessential amino acid tyrosine. The enzyme that carries out this reaction is the mixed function oxidase phenylalanine hydroxylase (PAH). Inherited deficiencies in PAH are associated with a condition known as phenylketonuria (PKU; see case 38). The absence of PAH results in elevations of phenylalanine and various phenylketones, the accumulation of which is associated with the neurologic defects seen in this disorder. PKU can be treated by controlling the dietary intake of phenylalanine. Diets low in phenylalanine will help prevent excessive elevations in phenylalanine. Phenylalanine cannot be completely eliminated from the diet because it is an essential amino acid needed for protein synthesis. In the absence of PAH activity, tyrosine becomes an essential amino acid because it cannot be generated from phenylalanine.
COMPREHENSION QUESTIONS
41.1 All amino acids are needed for the production of proteins in cells and for the synthesis of important biomolecules. Which of the following amino acids, all of which can be synthesized by the human body, must be taken in the diet because it is not synthesized in sufficient quantities to meet the body’s needs?
A. Asparagine
B. Glutamine
C. Methionine
D. Proline
E. Tyrosine
41.2 A young child is in an automobile accident that requires surgical intervention and substantial recovery time in the hospital. A consultation with a nutritionist results in a specific dietary plan. The plan included the supplementation of an amino acid that is typically considered a nonessential amino acid. Which of the following amino acids is an essential amino acid under conditions of enhanced growth or surgical recovery?
A. Alanine
B. Arginine
C. Glycine
D. Serine
E. Tyrosine
41.3 As part of a standard neonatal screen, an infant is diagnosed with a loss of function genetic defect in the enzyme phenylalanine hydroxylase. Defects in this enzyme can result in a condition known as PKU, which results from the toxic effects of phenylalanine-derived phenylketones. Fortunately, this condition can be managed by regulating the amount of phenylalanine provided in the diet. Which of the following nonessential amino acids will need to be supplied in the diet of this infant?
A. Alanine
B. Aspartate
C. Glycine
D. Serine
E. Tyrosine
ANSWERS
41.1 C. Methionine can be synthesized by the methylation of homocysteine by the enzyme methionine synthase, which requires the participation of vitamin B12 and 5-methyltetrahydrofolate. It is actually the homocysteine component of methionine that is required, since this reaction has the capacity to synthesize enough methionine. However, there is no good dietary source of homocysteine. This conversion of homocysteine to methionine also serves the purpose of making tetrahydrofolate (THF) available for other biosynthetic reactions.
41.2 B. Arginine is considered nonessential because biosynthetic pathways for its generation do exist in certain cells of the body. Arginine is synthesized from the amino acid glutamate. Glutamate is first converted to ornithine, which is then converted to arginine by enzymes of the urea cycle. The urea cycle is found only in the liver; therefore, the production of arginine through this pathway is limiting. The production of arginine through this pathway is likely sufficient for healthy adults, but it may not be sufficient in times of growth where increased protein synthesis augments the need for amino acids. Thus, in growing children and in adults following surgery, arginine becomes an essential amino acid.
41.3 E. Phenylalanine is an essential amino acid that is also used to generate the nonessential amino acid tyrosine. Because tyrosine is made from phenylalanine, it becomes an essential amino acid when phenylalanine levels are limited because of the absence of phenylalanine hydroxylase activity.
BIOCHEMISTRY PEARLS
![]() The term essential amino acid is used to identify those amino acids that must be taken in through the diet and cannot be manufactured.
The term essential amino acid is used to identify those amino acids that must be taken in through the diet and cannot be manufactured.
![]() There are 10 such essential amino acids for which biosynthetic pathways do not exist in cells of the human body.
There are 10 such essential amino acids for which biosynthetic pathways do not exist in cells of the human body.
![]() Phenylalanine is an essential amino acid that is also used to generate the nonessential amino acid tyrosine.
Phenylalanine is an essential amino acid that is also used to generate the nonessential amino acid tyrosine.
REFERENCE
Marks DB, Marks AD, Smith CM. Basic Medical Biochemistry: A Clinical Approach. Baltimore, MD: Lippincott Williams & Wilkins; 1996:569-646.