Case Files Biochemistry, 3rd Edition (2015)
SECTION II. Clinical Cases
CASE 45
A 65-year-old woman presents to the clinic feeling tired and fatigued all the time. She has also noticed an increasing problem with constipation despite adequate fiber intake. She is frequently cold when others are hot. Her skin has become dry, and she has noticed a swelling sensation in her neck area. On examination she is afebrile with a pulse of 60 beats/minute. She is in no acute distress and appears in good health. She has an enlarged, nontender thyroid noted on her neck. Her reflexes are diminished, and her skin is dry to the touch.
![]() What is the most likely diagnosis?
What is the most likely diagnosis?
![]() What laboratory test would you need to confirm the diagnosis?
What laboratory test would you need to confirm the diagnosis?
![]() What is the treatment of choice?
What is the treatment of choice?
ANSWERS TO CASE 45:
Hypothyroidism
Summary: A 65-year-old woman presents with weakness, fatigue, cold intolerance, constipation, dry skin, and goiter.
• Diagnosis: Hypothyroidism
• Laboratory tests: Thyroid-stimulating hormone (TSH) and free thyroxine (T4)
• Treatment: Thyroid hormone replacement with levothyroxine
CLINICAL CORRELATION
Hypothyroidism is quite common in older adults and may present with an indolent course, or it may induce dramatic mental changes such as coma or pericardial effusion with tamponade. The most common etiology is primary hypothyroidism, or failure of the thyroid gland to manufacture and release sufficient thyroid hormone. The diagnosis is established by an elevated TSH. The treatment is by thyroxine replacement.
APPROACH TO:
Thyroid Gland
OBJECTIVES
1. Describe thyroid hormone metabolism.
2. Explain the regulation of thyroid hormones.
3. Explain the role of iodine on synthesis of thyroid hormone.
DEFINITIONS
GRAVES DISEASE: An autoimmune disorder in which antibodies overstimulate the production of thyroid hormones leading to a condition of hyperthyroidism, or elevated thyroid hormone synthesis and secretion.
HASHIMOTO THYROIDITIS: An autoimmune disorder in which the thyroid gland is destroyed by the action of antibodies leading to a condition of hypothyroidism, or decreased thyroid hormone synthesis and secretion.
THYROID RESPONSE ELEMENTS (TREs): A domain on DNA that will bind the complex formed by thyroid hormone binding to the thyroid hormone receptor. When the complex binds to the TRE, which are located in the promoter region of the DNA, it activates transcription of the gene. When thyroid hormone is not bound to the receptor the receptor acts as a transcription repressor.
THYROXINE: T4; a thyroid hormone derived from the amino acid tyrosine that contains 4 iodine atoms per molecule.
TRH: Thyrotropin-releasing hormone; a tripeptide hormone that is released by the hypothalamus and that acts on the anterior pituitary to stimulate the release of thyroid-stimulating hormone.
TRIIODOTHYRONINE: T3; a thyroid hormone derived from the amino acid tyrosine that contains 3 iodine atoms per molecule.
TSH: Thyroid-stimulating hormone or thyrotropin; a glycoprotein hormone released from the anterior pituitary in response to increased levels of TRH. TSH binds to TSH receptors on the basal membrane of epithelial cells of the thyroid gland to stimulate the release of the thyroid hormones, T3 and T4.
DISCUSSION
The major circulating forms of thyroid hormone are thyroxine (T4), containing 4 iodine atoms per molecule, and triiodothyronine (T3), with 3 iodine atoms per molecule (Figure 45-1). Of these, T3 is eightfold more active. These are synthesized in the thyroid gland following TSH stimulation. TSH binds a G protein–coupled receptor to activate adenylate cyclase and trigger a signaling cascade leading to thyroid hormone biosynthesis. TSH is released from the pituitary in response to negative feedback by circulating levels of thyroid hormone as well as regulation by circulating levels of TRH, a tripeptide synthesized in the hypothalamus.
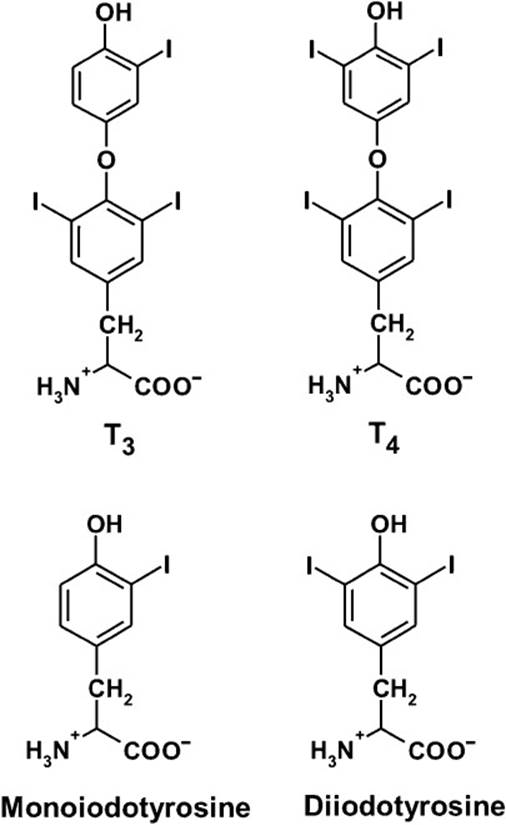
Figure 45-1. Structures of the thyroid hormones thyroxine (T4) and triiodothyronine (T3). Also shown are the intermediates monoiodotyrosine (MIT) and di-iodotyrosine (DIT) that are also formed on thyroglobulin.
Thyroid hormones are the only major biochemical species known to incorporate iodine. In fact, in third-world countries, iodine deficiency is the major cause of hypothyroidism (deficiency of thyroid hormones). Iodine deficiency is characterized by the development of a goiter, representing enlargement of the thyroid gland. In the developed world, where iodine deficiency is rare because of the use of iodized salt, autoimmune disorders are a leading cause of thyroid disease. These are characterized by the presence of antibodies in the blood that either stimulate or damage the thyroid gland. The most common examples are Graves disease, characterized by antibody overstimulation of thyroid hormone production, and Hashimoto thyroiditis, leading to autoimmune destruction of the thyroid gland. In addition, inherited human disorders resulting in mutations in the thyroid hormone receptor, which may abolish hormone binding, have been reported. These individuals exhibit symptoms of hypothyroidism as well as a high incidence of attention-deficit disorder. This trait is genetically dominant, indicating that the mutant receptors act in a dominant negative manner.
Thyroid hormone biosynthesis (Figure 45-2) involves the concentrative uptake of iodide into thyroid cells where it is converted into iodine by thyroid peroxidase in the colloid space of the follicular lumen. Iodine is incorporated into tyrosine residues of thyroglobulin contained within the colloid space at the basal surface of the thyroid follicular cell. Tyrosine residues are iodinated at either one or 2 sites, and then these residues are coupled to generate either T3 or T4 residues within thyroglobulin. The iodinated thyroglobulin is then taken up from the extracellular matrix into the cytoplasm of the thyroid cell where lysosomal proteases cleave T3 and T4 from thyroglobulin. The hormones are then carried in the blood bound primarily to thyroid-binding globulin. T4 is converted to T3 in the liver and to a lesser extent other tissues, accounting for 80% of circulating T3.
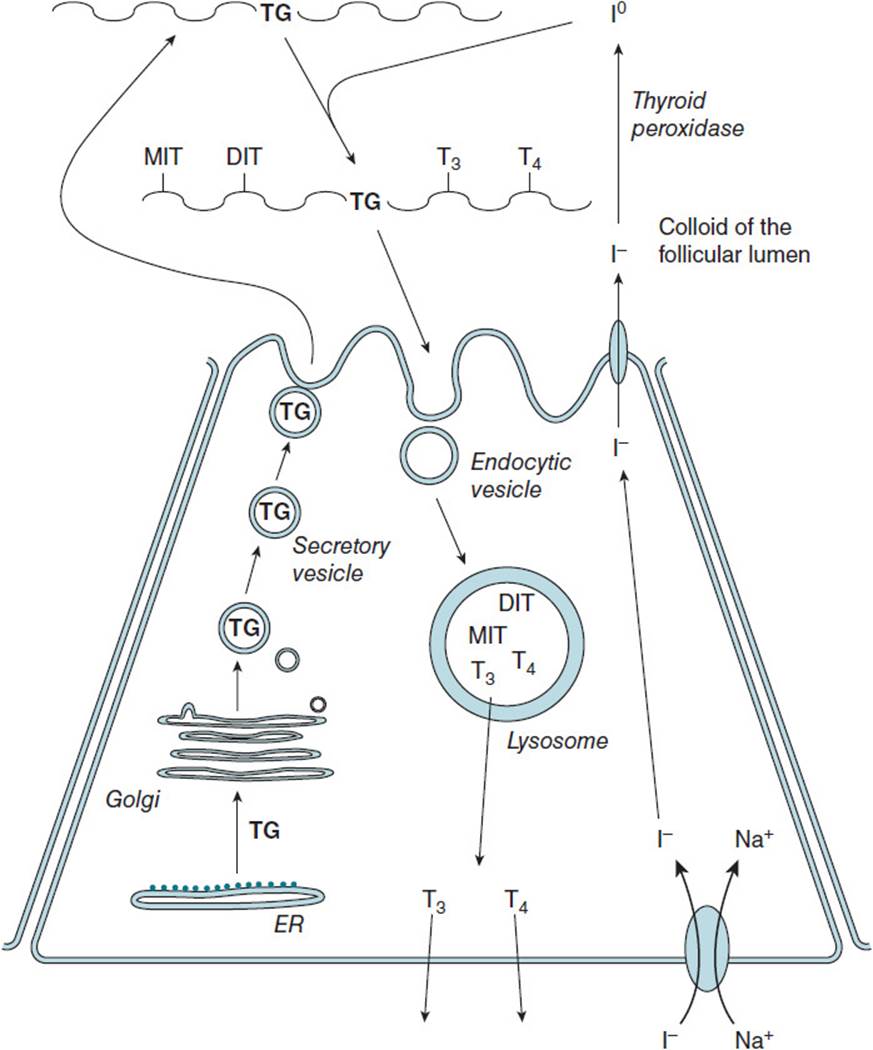
Figure 45-2. Biosynthesis of the thyroid hormones T3 and T4 in the thyroid follicular cell and release into the bloodstream. DIT = diiodotyrosine, MIT = monoiodotyrosine, T3 = triiodothyronine, T4 = thyroxine, TG = thyroglobulin.
Thyroid hormones stimulate protein synthesis in most cells of the body. They also stimulate oxygen consumption by increasing the levels of the Na+, K+-ATPase ion transporter. The generation of plasma membrane Na+and K+ gradients by the Na+, K+-ATPase is a major consumer of cellular adenosine triphosphate (ATP), leading to stimulation of ATP synthesis in the mitochondria thus directly increasing mitochondrial energy metabolism. By this means, thyroid hormones aid the conversion of food into energy and heat. In all of its known actions, thyroid hormone exerts its effects by interaction with its receptor in the cell nucleus and activation of transcription of the target genes.
Thyroid hormone receptors are members of a large nuclear receptor super-family that includes receptors for steroid hormones, vitamin D3, and retinoic acid. Members of this receptor superfamily contain a DNA-binding domainresponsible for binding to hormone response elements contained within the promoters of target genes. In addition, members of this superfamily contain a region responsible for specific binding to the hormone or biologically active agent. DNA-binding specificity is mediated by receptor sequence motifs known as zinc fingers, owing to their chelation of one zinc ion per loop, or finger. In the case of thyroid hormone, receptors bind thyroid response elements (TREs). Thyroid hormone receptors can bind TREs as monomers, as homodimers, or as heterodimers with the retinoid X receptor, another member of this superfamily. The latter exhibits the highest DNA-binding affinity and is the major functional form of the receptor.
In essence, these receptors serve as hormone-activated transcription factors that directly regulate transcription of messenger RNA (mRNA) from target genes. By contrast to other members of this superfamily, thyroid hormone receptors bind their sites on the promoter regions of DNA in the absence of bound hormone, usually resulting in transcriptional repression. Binding of thyroid hormone triggers a conformational change in the receptor, converting it to a transcriptional activator. In this state, it is competent to bind a group of coactivator proteins including histone transacetylase, an activity that serves to create a more open configuration on adjacent chromatin. Mammalian thyroid receptors are encoded by 2 different genes, each of which can be alternatively spliced, and, thus, yield 4 different receptor isoforms. These isoforms differ in their functional characteristics as well as their tissue-specific and developmental stage–dependent expression, underscoring the complexity of the multiple physiologic effects of thyroid hormones.
COMPREHENSION QUESTIONS
45.1 A 25-year-old woman sought treatment for her constant fatigue, lethargy, and depression. She was small in stature and had previously been diagnosed with attention-deficit/hyperactivity disorder. On physical examination she was found to have an enlarged thyroid gland (goiter). Blood tests revealed elevated levels of T3, T4, and TSH, yet she did not exhibit typical symptoms of hyperthyroidism. Which one of the following possibilities offers the most likely explanation of her symptoms?
A. Thyroid hormone overproduction because of a thyroid gland tumor
B. Hypersecretion of TSH because of a pituitary tumor
C. Genetic alteration in the thyroid receptor reducing its ability to bind thyroid hormone
D. Mutation in the TSH receptor in the thyroid gland reducing its ability to bind TSH
E. Iodide deficiency in the diet
45.2 In individuals with iodide deficiency, which one of the following is most likely?
A. TSH levels are elevated and directly stimulate growth of the thyroid gland to a very large size.
B. Mono- and diiodinated thyroid hormone molecules are produced, and elevated levels of these derivatives compensate for the deficiency.
C. TSH levels are decreased, relieving their inhibitory effects on thyroid cell proliferation.
D. Synthesis of the Na+, K+-ATPase is increased.
E. Tissue utilization of oxygen is increased.
45.3 In women taking thyroid hormone replacement pills, the dosage must be adjusted if they start taking birth control pills. Which one of the following best explains this situation?
A. Thyroid hormones block the action of estrogens, so the estrogen dose must be increased.
B. Estrogens block the action of thyroid hormones, so the dose of thyroid hormone must be increased.
C. Progestins block the action of thyroid hormone, so the dose of thyroid hormone must be increased.
D. Thyroid hormones stimulate the action of estrogens, so the estrogen dose must be decreased.
E. Estrogens stimulate the action of thyroid hormone, so the dose of thyroid hormone must be decreased.
ANSWERS
45.1 C. The patient exhibits symptoms of hypothyroidism including goiter, yet thyroid hormone levels are elevated. This pattern can only be explained by resistance of target cells to thyroid hormone, for example, a mutation of the receptor decreasing its binding affinity for hormone. Iodide deficiency would lead to goiter but not increased hormone levels.
45.2 A. Elevation of TSH is the mechanism for goiter formation. Decreased thyroid hormone levels reduce feedback inhibition of TSH secretion by the pituitary. Therefore, TSH secretion is increased. TSH acts as a growth factor for the thyroid gland, increasing its mass and, thus, its capacity to synthesize thyroid hormones.
45.3 B. Estrogens partially block the action of thyroid hormones, making them less effective.
BIOCHEMISTRY PEARLS
![]() The major circulating forms of thyroid hormone are T4, containing 4 iodine atoms per molecule, and tri-iodothyronine (T3), with 3 iodine atoms per molecule.
The major circulating forms of thyroid hormone are T4, containing 4 iodine atoms per molecule, and tri-iodothyronine (T3), with 3 iodine atoms per molecule.
![]() TSH binds a G protein–coupled receptor to activate adenylate cyclase and trigger a signaling cascade leading to thyroid hormone biosynthesis.
TSH binds a G protein–coupled receptor to activate adenylate cyclase and trigger a signaling cascade leading to thyroid hormone biosynthesis.
![]() In developed countries, where iodine deficiency is rare because of the use of iodized salt, autoimmune disorders are a leading cause of thyroid disease.
In developed countries, where iodine deficiency is rare because of the use of iodized salt, autoimmune disorders are a leading cause of thyroid disease.
![]() Thyroid hormone receptors bind their sites on the promoter regions of DNA in the absence of bound hormone, usually resulting in transcriptional repression.
Thyroid hormone receptors bind their sites on the promoter regions of DNA in the absence of bound hormone, usually resulting in transcriptional repression.
![]() Binding of thyroid hormone triggers a conformational change in the receptor, converting it to a transcriptional activator.
Binding of thyroid hormone triggers a conformational change in the receptor, converting it to a transcriptional activator.
REFERENCES
Barrett EJ. The thyroid gland. In: Boron WF, Boulpaep EL, eds. Medical Physiology: A Cellular and Molecular Approach. 2nd ed. Philadelphia, PA: W.B. Saunders; 2011.
Bowen RA, Austgen L, Rouge M. Pathophysiology of the Endocrine System. Colorado State University, 2006. http://arbl.cvmbs.colostate.edu/hbooks/pathphys/endocrine/.
Litwack G, Schmidt TJ. Biochemistry of hormones I: polypeptide hormones. In: Devlin TM, ed. Textbook of Biochemistry with Clinical Correlations. 7th ed. New York: Wiley-Liss; 2010.