Case Files Biochemistry, 3rd Edition (2015)
SECTION II. Clinical Cases
CASE 3
A 1-month-old baby girl is brought to the emergency department (ED) by her mom with concerns over her baby’s decreased activity level, lack of appetite, and worsening diaper rash that has not resolved with usual antifungal treatment. The baby was born at term and mother denies any pregnancy or delivery complications. The baby was seen by the pediatrician at 2 wks and was doing okay but has had frequent upper respiratory infection symptoms. The mother denies any fever or nausea/vomiting in her newborn. On examination, the baby is afebrile but is noted to be lethargic with no lymphadenopathy. Mild hepatosplenomegaly and hypotonia are noted on physical examination. Chest radiography demonstrates an absent thymic shadow but normal heart silhouette. Laboratory values revealed elevated levels of adenosine and deoxyadenosine in the urine and blood.
![]() What is the likely diagnosis?
What is the likely diagnosis?
![]() What is the biochemical basis for this disorder?
What is the biochemical basis for this disorder?
![]() What are potential treatment options for this baby?
What are potential treatment options for this baby?
ANSWERS TO CASE 2:
A 32-year-old woman is being treated with methotrexate for a recently diagnosed choriocarcinoma of the ovary and presents with complaints of oral mucosal ulcers. The patient recalls being advised not to take folate-containing vitamins during therapy. An uncomplicated surgical exploration was performed 5 weeks ago with removal of the affected ovary. The patient has been taking methotrexate for 2 weeks and has never had any of the above symptoms before. On examination, patient was afebrile and appeared ill. Several mucosal ulcers were seen in her mouth. The patient also had some upper abdominal tenderness. Her platelet count is decreased at 60,000/mm3(normal, 150,000–450,000/mm3).
![]() What is the most likely etiology of her symptoms?
What is the most likely etiology of her symptoms?
![]() What is the biochemical explanation of her symptoms?
What is the biochemical explanation of her symptoms?
![]() What part of the cell cycle does methotrexate act on?
What part of the cell cycle does methotrexate act on?
ANSWERS TO CASE 3:
Methotrexate and Folate Metabolism
Summary: A 32-year-old woman has oral ulcerations and thrombocytopenia (low platelet count) after beginning methotrexate initiated for recently diagnosed ovarian cancer. She recalls being instructed to avoid folate during therapy.
• Likely cause of her symptoms: Adverse events of methotrexate (antimetabolite chemotherapy) affecting rapidly dividing cells such as oral mucosa.
• Biochemical explanation of her symptoms: Related to effects of methotrexate on cell cycle of all cells (particularly rapidly dividing cells). Folate antagonists inhibit dihydrofolate reductase (tetrahydrofolate needed for purine synthesis).
• Cell cycle affected by methotrexate: DNA synthesis (S) phase.
CLINICAL CORRELATION
Chemotherapeutic agents are used to treat various types of cancers. Although some are specific for cancer cells, most chemotherapeutic agents are toxic for both normal and cancer cells. Methotrexate acts as a folate antagonist, affecting DNA synthesis. Because cancer cells divide faster than normal cells, a higher proportion of these neoplastic cells will die. Nevertheless, normal cells that also are rapidly dividing, such as the gastrointestinal mucosa, the oral mucosa, and the bone marrow cells, may be affected. The patient was advised to avoid folate during therapy, since folate would be an “antidote,” and would allow the cancer cells to escape cell kill.
APPROACH TO:
Cell Cycle
OBJECTIVES
1. Understand the components of the cell cycle.
2. Know how folate is involved in DNA synthesis.
3. Be familiar with the terminology of nucleoside and nucleotide.
DEFINITIONS
CELL CYCLE: The time interval between cell divisions in proliferating cells. The cell cycle is divided into 4 phases: M phase, in which mitosis takes place; G1 phase, prior to synthesis of DNA; S phase, in which DNA and histones are synthesized to duplicate the chromosomes; and G2 phase, during which there is cell growth and synthesis of macromolecules. Under certain conditions, the cell can enter a quiescent, or G0 phase, which is not part of the regular cell cycle.
DIHYDROFOLATE REDUCTASE (DHFR): The enzyme required to convert folic acid to its active form, tetrahydrofolate. It requires the cofactor NADPH as a source of reducing equivalents to reduce folate first to dihydrofolate (DHF) and then to tetrahydrofolate.
METHOTREXATE: A drug that has a similar structure to DHFR. It binds to DHFR reductase and competitively inhibits it, thus decreasing the levels of tetrahydrofolate in the cells. It effectively stops DNA synthesis in rapidly dividing cells such as cancer cells.
TETRAHYDROFOLATE (THF): The active form of the vitamin folic acid. THF is one of the major carriers of one-carbon units at various oxidation states for biosynthetic reactions. It is required for the synthesis of the nucleotide thymidylate (dTMP). Although bacteria can synthesize folic acid, eukaryotes must obtain folate from the diet. Dietary sources of folate include leafy green vegetables (eg, spinach and turnip greens), citrus fruits, and legumes. Many breakfast cereals, breads, and other grain products are fortified with folate.
DNA: Two large molecules composed of deoxynucleotides attached by hydrogen bonds in a helical, antiparallel relationship.
NUCLEOSIDES: Nitrogenous base plus a sugar (bases in DNA are adenine [A], thymine [T], cytosine [C], and guanine [G]; bases in RNA are A, C, G, but uracil [U] instead of T; sugar moiety in DNA is deoxyribose, and in RNA is ribose).
NUCLEOTIDE: Nucleoside plus phosphate group (Table 3-1).
Table 3-1 • TERMINOLOGY OF NUCLEIC ACIDS
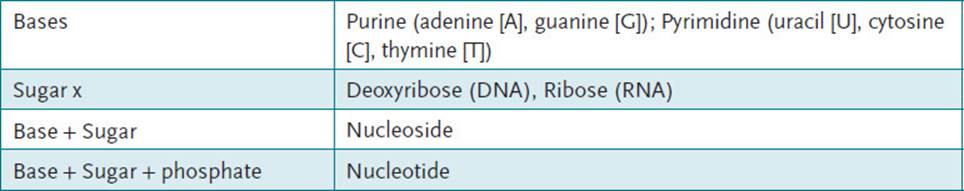
DEOXYTHYMIDYLATE (DTMP): DNA nucleotide consisting of the deoxyribose sugar, thymidine as nitrogenous base, and 1 phosphate.
DISCUSSION
The cell cycle is defined as the time interval between cell divisions in proliferating cells. It is important to note that the cell cycle is not a simple “clock.” Movement through the cell cycle is controlled by a variety of proteins that allow the cell to respond to various stimuli. The eukaryote cell cycle is composed of the following 4 phases (Figure 3-1):
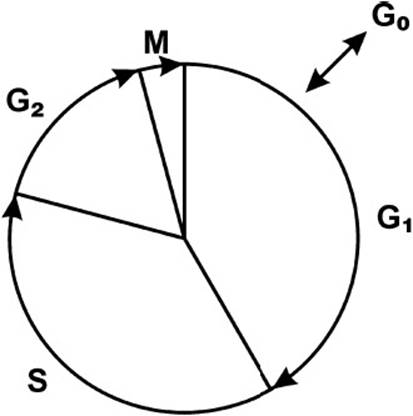
Figure 3-1. Cell cycle. G0 = rest phase, G1 = cell growth, G2 = proteins made in preparation for cell division, M = mitosis, S = DNA synthesis and replication.
1. M phase: Mitosis
2. G1 phase (gap 1): Between mitosis and initiation of DNA synthesis
3. S phase: When DNA synthesis occurs
4. G2 phase (gap 2): Cell growth and macromolecule synthesis
Although great variation exists in the length of the mammalian cell cycle (hours to days), as a generalization, mammalian cells divide once every 24 hours. The M and S phases of the cell cycle are relatively constant. Therefore, the length of the mammalian cell cycle is determined by length of the G1 and G2 phases. Cell division occurs during M phase, and the mitotic interphase is composed of (G1 + S + G2). M phase, or mitosis, is divided into 4 subphases: prophase, metaphase, anaphase, and telophase. In prophase, the nuclear membrane breaks down while the replicated chromosomes condense and are released into the cytoplasm. The chromosomes become aligned on the equatorial plate of the cell during metaphase, and they move from the equatorial plate to the poles during anaphase. The final step in M phase, telophase, is reformation of the nuclear membrane around the chromosomes followed by cytokinesis, or formation of two daughter cells. During the G1 phase, the cell “monitors” itself and its environment. The cell is metabolically active and undergoes continuous growth, but no DNA synthesis occurs during this phase. During G1 the cell makes a “decision” either to continue in the cell cycle and divide or to “withdraw” from the cell cycle and differentiate. The synthesis of DNA and histones to form two sets of chromosomes occurs during S phase. The key event of the G2 phase is for the cell to “make sure that its entire DNA has replicated.” Continued cell growth and the synthesis of cellular macromolecules also occur during the G2 phase, preparing for cell division.
Under certain conditions cells can leave G1 and enter into the G0 phase of the cell cycle. This phase is not part of the regular cell cycle and represents a specialized state, which can be temporary or permanent. Entry into the G0phase can be triggered by growth factor withdrawal, negative growth factors, or limited protein synthesis. The cells in G0 are in a nonproliferative or quiescent state, which can vary tremendously in length. Some cells differentiate and never divide again. Others can resume proliferation to replace lost cells as a result of injury or cell death. An important point is that cancer cells do not generally have a G0 phase.
Key regulatory proteins that govern the cell cycle are the cyclin-dependent kinases (CDKs), which are serine/threonine protein kinases, and the cyclins, which are regulatory proteins that bind to the CDKs. Proteins that inhibit the kinase activity (cyclin-dependent kinase inhibitors [CDIs]) are also present in the cell. The CDKs are regulated by the levels of cyclins and the CDK/cyclin complex phosphorylates proteins on serine or threonine residues. The cyclins bind to and activate CDKs via protein–protein interactions. Cyclins act as regulatory subunits controlling activity and specificity of the CDK activity. These cyclins can themselves be phosphorylated and dephosphorylated. Cyclin accumulation and degradation controls normal cell cycle activity. For example, the degradation of specific cyclins by proteolysis at the metaphase-anaphase transition ends mitosis.
THF is the major source of 1-carbon units used in the biosynthesis of many important biologic molecules. This cofactor is derived from the vitamin folic acid and is a carrier of activated 1-carbon units at various oxidation levels (methyl, methylene, formyl, formimino, and methenyl). These compounds can be interconverted as required by the cellular process. The major donor of the 1-carbon unit is serine in the following reaction:
![]()
All cells, especially rapidly growing cells, must synthesize thymidylate (dTMP) for DNA synthesis. The difference between (T) and (U) is one methyl group at the carbon-5 position. Thymidylate is synthesized by the methylation of uridylate (dUMP) in a reaction catalyzed by the enzyme thymidylate synthase. This reaction requires a methyl donor and a source of reducing equivalents, which are both provided by N5, N10-methylene THF (Figure 3-2). For this reaction to continue, the regeneration of THF from dihydrofolate (DHF) must occur.
![]()
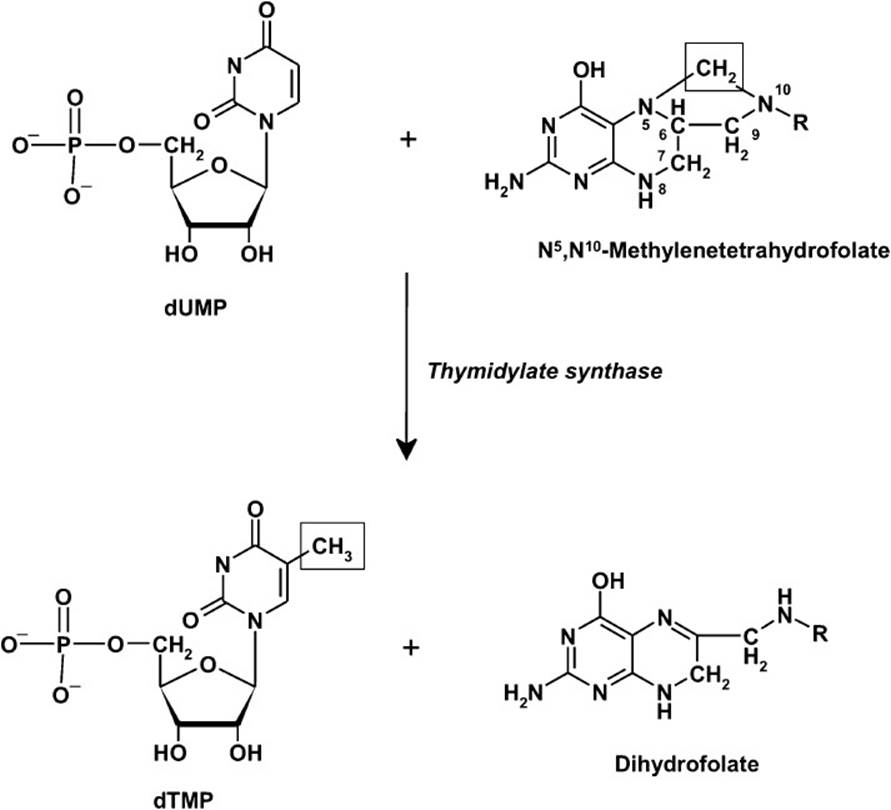
Figure 3-2. Thymidylate synthesized by the methylation of uridylate (dUMP) in a reaction catalyzed by the enzyme thymidylate synthase.
The enzyme dihydrofolate reductase (DHFR) catalyzes this reaction, which is a target of the anticancer drugs aminopterin and methotrexate. These drugs are analogs of DHF and act as competitive inhibitors of DHFR.Inhibition of this enzyme prevents the regeneration of THF and blocks dTMP synthesis because of the lack of the methyl donor required for the reaction of thymidylate synthase.
THF is also required as a donor of 2 carbon atoms in the synthesis of the purine ring structure required for adenine and guanine. The carbon atoms donated by THF are indicated in Figure 3-3. Therefore, a lack of THF blocks the synthesis of the purine ring structure because of the lack of the ability of the cell to synthesize N10-formyl-THF.
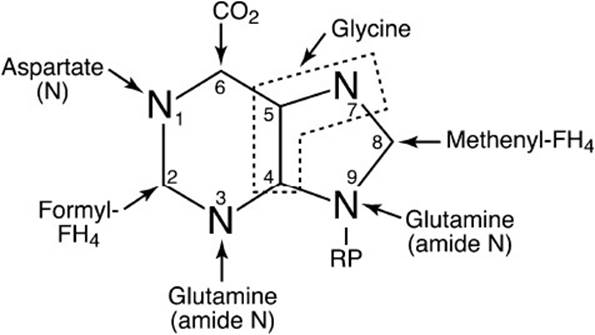
Figure 3-3. Origin of the atoms of the purine base ring system. RP = pentose phosphate.
In summary, DNA synthesis requires synthesis of dTMP and the purines adenine and guanine. THF, derived from the vitamin folic acid, is required for the biosynthesis of these nucleotides. Treatment with methotrexate blocks the ability of the cell to regenerate THF, leading to inhibition of these biosynthetic pathways. The lack of nucleotides prevents DNA synthesis, and these cancer cells cannot divide without DNA synthesis. Unfortunately, the effects of methotrexate are nonspecific and other rapidly dividing cells, such as epithelial cells in the oral cavity, intestine, skin, and blood cells, are also inhibited. This leads to the adverse events associated with methotrexate (and other cancer chemotherapy drugs) such as mouth sores, low white blood cell counts, stomach upset, hair loss, skin rashes, and itching.
COMPREHENSION QUESTIONS
3.1 A 44-year-old woman who recently lost her job because of absenteeism presents to her physician complaining of loss of appetite, fatigue, muscle weakness, and emotional depression. The physical examination reveals a somewhat enlarged liver that feels firm and nodular, and there is a hint of jaundice in the sclerae and a hint of alcohol on her breath. The initial laboratory profile included a hematologic analysis that showed that she had an anemia with enlarged red blood cells (macrocytic). A bone marrow aspirate confirmed the suspicion that she has a megaloblastic anemia because it showed a greater than normal number of red and white blood cell precursors, most of which were larger than normal. Further analyses revealed that her serum folic acid level was 2.9 ng/mL (normal = 6 to 15), her serum B12 level was 153 pg/mL (normal = 150 to 750), and her serum iron level was normal. Megaloblastic anemia in this patient is most likely caused by which of the following?
A. Decreased synthesis of methionine
B. Decreased conversion of dUMP to dTMP
C. Decrease in the synthesis of phosphatidyl choline
D. Decrease in the levels of succinyl CoA
E. Decreased synthesis of dUTP
3.2 A patient presents with a urinary tract infection and is prescribed a combination drug containing trimethoprim and sulfamethoxazole. These drugs are effective because they do which of the following?
A. Bind to operons to prevent synthesis of bacterial messenger RNA
B. Block transport across bacterial cell walls
C. Inhibit bacterial synthesis of cobalamin (B12)
D. Inhibit bacterial synthesis of THF
E. Inhibit synthesis of phospholipids in bacteria
3.3 Methotrexate is often used as a chemotherapeutic agent to treat patients with leukemia. This drug is effective because it inhibits cells in which part of the cell cycle?
A. G1 phase
B. S phase
C. M phase
D. G2 phase
E. G0 phase
3.4 Leukemia patients are often given the compound leucovorin (N5-formyl THF) following treatment with the drug methotrexate. What is the mechanism whereby Leucovorin is useful as part of this treatment protocol?
A. It facilitates the uptake of methotrexate by cells.
B. It can be converted to THF by bypassing DHFR.
C. It acts as an activator of thymidylate synthase.
D. It prevents the uptake of methotrexate by normal cells.
E. It stimulates cells of the immune system.
ANSWERS
3.1 B. Megaloblastic anemia is caused by a decrease in the synthesis of deoxythymidylate and the purine bases usually caused by a deficiency in either THF or cobalamin or both. This results in decreased DNA synthesis, which results in abnormally large hematopoietic cells created by perturbed cell division and DNA replication and repair. This patient exhibits signs of chronic alcoholism, which often leads to a folate deficiency. This can occur due to poor dietary intake, decreased absorption of folate due to damage of the intestinal brush border cells and resulting conjugase deficiency, and poor renal resorption of folate.
3.2 D. Bacteria must synthesize the folate required for their biosynthetic processes; they do not have a transporter to bring folate into the cell. Trimethoprim inhibits prokaryotic DHFR (eukaryotic is not affected) and sulfamethoxazole is an analog of p-aminobenzoic acid (PABA), a precursor to folic acid. Bacteria will use this analog instead of PABA and produce a nonfunctional folate.
3.3 B. Methotrexate inhibits the synthesis of deoxythymidine by preventing the regeneration of THF by inhibiting the enzyme DHFR. Inhibiting the synthesis of deoxythymidylate prevents the cell from synthesizing its DNA. DNA synthesis occurs exclusively during S phase of the cell cycle.
3.4 B. Leucovorin (N5-formyl THF, folinic acid) is used as an antidote for cells that have decreased levels of folic acid. Treatment of leukemia patients with methotrexate kills not only the tumor cells but also other normal rapidly dividing cells. N5-formyl THF is normally administered 24 hours following treatment with methotrexate; it can be converted to THF by these normal cells by bypassing the block caused by methotrexate. Therefore, these normal cells can synthesize deoxythymidine and carry out DNA synthesis.
BIOCHEMISTRY PEARLS
![]() The eukaryote cell cycle is composed of the following 4 phases: M phase, mitosis; G1 phase (gap 1), between mitosis and initiation of DNA synthesis; S phase, DNA synthesis; and G2 phase (gap 2), cell growth and macromolecule synthesis.
The eukaryote cell cycle is composed of the following 4 phases: M phase, mitosis; G1 phase (gap 1), between mitosis and initiation of DNA synthesis; S phase, DNA synthesis; and G2 phase (gap 2), cell growth and macromolecule synthesis.
![]() The cells in G0 are in a nonproliferative or quiescent state that can vary tremendously in length.
The cells in G0 are in a nonproliferative or quiescent state that can vary tremendously in length.
![]() DNA synthesis requires the formation of dTMP and the purines adenine and guanine. THF, derived from the vitamin folic acid, is required for the biosynthesis of these nucleotides.
DNA synthesis requires the formation of dTMP and the purines adenine and guanine. THF, derived from the vitamin folic acid, is required for the biosynthesis of these nucleotides.
![]() The cell cycle is regulated by the specific proteins CDKs, which are serine/threonine protein kinases, and the cyclins, which are regulatory proteins that bind to the CDKs.
The cell cycle is regulated by the specific proteins CDKs, which are serine/threonine protein kinases, and the cyclins, which are regulatory proteins that bind to the CDKs.
![]() Treatment with methotrexate blocks the ability of the cell to regenerate THF, leading to inhibition of these biosynthetic pathways. Thus, methotrexate affects DNA synthesis.
Treatment with methotrexate blocks the ability of the cell to regenerate THF, leading to inhibition of these biosynthetic pathways. Thus, methotrexate affects DNA synthesis.
REFERENCES
Alberts B, Johnson A, Lewis J, et al. The cell cycle and programmed cell death. Molecular Biology of the Cell. 5th ed. New York: Garland; 2007.
Cory JG. Purine and pyrimidine nucleotide metabolism. In: Devlin TM, ed. Textbook of Biochemistry with Clinical Correlations. 7th ed. New York: Wiley-Liss; 2010.