Harper’s Illustrated Biochemistry, 29th Edition (2012)
SECTION I. Structures & Functions of Proteins & Enzymes
Chapter 9. Enzymes: Regulation of Activities
Peter J. Kennelly, PhD & Victor W. Rodwell, PhD
OBJECTIVES
After studying this chapter, you should be able to:
![]() Explain the concept of whole-body homeostasis and its response to fluctuations in the external environment.
Explain the concept of whole-body homeostasis and its response to fluctuations in the external environment.
![]() Discuss why the cellular concentrations of substrates for most enzymes tend to be close to Km.
Discuss why the cellular concentrations of substrates for most enzymes tend to be close to Km.
![]() List multiple mechanisms by which active control of metabolite flux is achieved.
List multiple mechanisms by which active control of metabolite flux is achieved.
![]() Describe the advantages of certain enzymes being elaborated as proenzymes.
Describe the advantages of certain enzymes being elaborated as proenzymes.
![]() Illustrate the physiologic events that trigger the conversion of a proenzyme to the corresponding active enzyme.
Illustrate the physiologic events that trigger the conversion of a proenzyme to the corresponding active enzyme.
![]() Describe typical structural changes that accompany conversion of a proenzyme to the active enzyme.
Describe typical structural changes that accompany conversion of a proenzyme to the active enzyme.
![]() Describe the basic features of a typical binding site for metabolites and second messengers that regulate catalytic activity of certain enzymes.
Describe the basic features of a typical binding site for metabolites and second messengers that regulate catalytic activity of certain enzymes.
![]() Indicate two general ways in which an allosteric effector can modify catalytic activity.
Indicate two general ways in which an allosteric effector can modify catalytic activity.
![]() Outline the roles of protein kinases, protein phosphatases, and of regulatory and hormonal and second messengers in initiating a metabolic process.
Outline the roles of protein kinases, protein phosphatases, and of regulatory and hormonal and second messengers in initiating a metabolic process.
BIOMEDICAL IMPORTANCE
The nineteenth-century physiologist Claude Bernard enunciated the conceptual basis for metabolic regulation. He observed that living organisms respond in ways that are both quantitatively and temporally appropriate to permit them to survive the multiple challenges posed by changes in their external and internal environments. Walter Cannon subsequently coined the term “homeostasis” to describe the ability of animals to maintain a constant intracellular environment despite changes in their external environment. We now know that organisms respond to changes in their external and internal environment by balanced, coordinated adjustments in the rates of specific metabolic reactions. Perturbations of the sensor-response machinery responsible for maintaining homeostatic balance can be deleterious to human health. Cancer, diabetes, cystic fibrosis, and Alzheimer’s disease, for example, are all characterized by regulatory dysfunctions triggered by pathogenic agents or genetic mutations. Many oncogenic viruses elaborate protein-tyrosine kinases that modify the regulatory events that control patterns of gene expression, contributing to the initiation and progression of cancer. The toxin from Vibrio cholerae, the causative agent of cholera, disables sensor-response pathways in intestinal epithelial cells by ADP-ribosylating the GTP-binding proteins (G-proteins) that link cell surface receptors to adenylyl cyclase. The consequent activation of the cyclase leads to the unrestricted flow of water into the intestines, resulting in massive diarrhea and dehydration. Yersinia pestis, the causative agent of plague, elaborates a protein-tyrosine phosphatase that hydrolyzes phosphoryl groups on key cytoskeletal proteins. Dysfunctions in the proteolytic systems responsible for the degradation of defective or abnormal proteins are believed to play a role in neurodegenerative diseases such as Alzheimer and Parkinson’s. In addition to their immediate function as regulators of enzyme activity, protein degradation, etc, covalent modifications such as phosphorylation, acetylation, and ubiquitination provide a protein-based code for the storage and hereditary transmission of information (Chapter 35). Such DNA-independent information systems are referred to as epigenetic.Knowledge of factors that control the rates of enzyme-catalyzed reactions thus is essential to an understanding of the molecular basis of disease and its transmission. This chapter outlines the patterns by which metabolic processes are controlled, and provides illustrative examples. Subsequent chapters provide additional examples.
REGULATION OF METABOLITE FLOW CAN BE ACTIVE OR PASSIVE
Enzymes that operate at their maximal rate cannot respond to increases in substrate concentration, and can respond only to precipitous decreases in substrate concentration. The Km values for most enzymes, therefore, tend to be close to the average intracellular concentration of their substrates, so that changes in substrate concentration generate corresponding changes in the metabolite flux (Figure 9–1). Responses to changes in substrate level represent an important but passive means for coordinating metabolite flow and maintaining homeostasis in quiescent cells. However, they offer a limited scope for responding to changes in environmental variables. The mechanisms that regulate enzyme efficiency in an active manner in response to internal and external signals are discussed below.
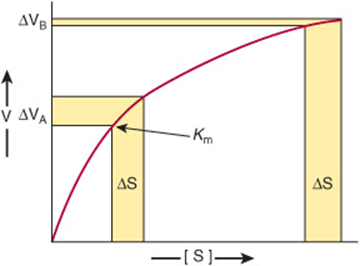
FIGURE 9–1 Differential response of the rate of an enzyme-catalyzed reaction, ΔV, to the same incremental change in substrate concentration at a substrate concentration close to Km (ΔVA) or far above Km (ΔVB).
Metabolite Flow Tends to Be Unidirectional
Despite the existence of short-term oscillations in metabolite concentrations and enzyme levels, living cells exist in a dynamic steady state in which the mean concentrations of metabolic intermediates remain relatively constant over time. While all chemical reactions are to some extent reversible, in living cells the reaction products serve as substrates for—and are removed by—other enzyme-catalyzed reactions (Figure 9–2). Many nominally reversible reactions thus occur unidirectionally. This succession of coupled metabolic reactions is accompanied by an overall change in free energy that favors unidirectional metabolite flow (Chapter 11). The unidirectional flow of metabolites through a pathway with a large overall negative change in free energy is analogous to the flow of water through a pipe in which one end is lower than the other. Bends or kinks in the pipe simulate individual enzyme-catalyzed steps with a small negative or positive change in free energy. Flow of water through the pipe nevertheless remains unidirectional due to the overall change in height, which corresponds to the overall change in free energy in a pathway (Figure 9–3).
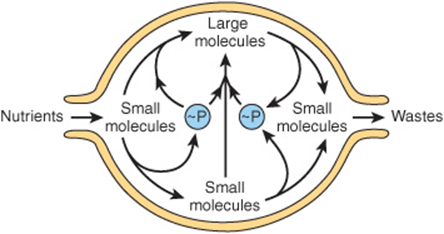
FIGURE 9–2 An idealized cell in steady state. Note that metabolite flow is unidirectional.
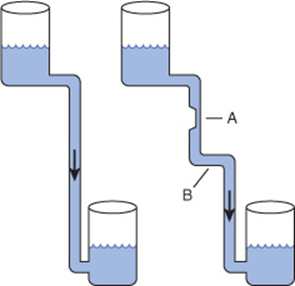
FIGURE 9–3 Hydrostatic analogy for a pathway with a rate-limiting step (A) and a step with a ΔG value near 0 (B).
COMPARTMENTATION ENSURES METABOLIC EFFICIENCY & SIMPLIFIES REGULATION
In eukaryotes, anabolic and catabolic pathways that inter-convert common products may take place in specific subcellular compartments. For example, many of the enzymes that degrade proteins and polysaccharides reside inside organelles called lysosomes. Similarly, fatty acid biosynthesis occurs in the cytosol, whereas fatty acid oxidation takes place within mitochondria (Chapters 22 and 23). Segregation of certain metabolic pathways within specialized cell types provides a further means for physical compartmentation.
Fortunately, many apparently antagonistic pathways can coexist in the absence of physical barriers, provided that thermodynamics dictates that each proceeds with the formation of one or more unique intermediates. For any reaction or series of reactions, the change in free energy that takes place when metabolite flow proceeds in the “forward” direction is equal in magnitude but opposite in sign from that required to proceed in the reverse direction. Some enzymes within these pathways catalyze reactions, such as isomerizations, that can act as bidirectional catalysts in vivo because the difference in free energy between substrates and products is close to zero. However, they represent the exception rather than the rule. Virtually all metabolic pathways proceed via one or more steps for which ΔG is significant. For example glycolysis, the breakdown of glucose to form two molecules of pyruvate, has a favorable overall ΔG of –96 kJ/mol, a value much too large to simply operate in “reverse” when wishing to convert excess pyruvate to glucose. Consequently, gluconeogenesis proceeds via a pathway in which the three most energetically disfavored steps from glycolysis are replaced by new reactions catalyzed by distinct enzymes (Chapter 20).
The ability of enzymes to discriminate between the structurally similar coenzymes NAD+ and NADP+ also results in a form of compartmentation. The reduced forms of both coenzymes are not readily distinguishable. However, the reactions that generate and later consume electrons that are destined for ATP generation are segregated in NADH, away from those used in the reductive steps of many biosynthetic pathways, which are carried by NADPH.
Controlling an Enzyme That Catalyzes a Rate-Limiting Reaction Regulates an Entire Metabolic Pathway
While the flux of metabolites through metabolic pathways involves catalysis by numerous enzymes, active control of homeostasis is achieved by the regulation of only a select subset of these enzymes. The ideal enzyme for regulatory intervention is one whose quantity or catalytic efficiency dictates that the reaction it catalyzes is slow relative to all others in the pathway. Decreasing the catalytic efficiency or the quantity of the catalyst responsible for the “bottleneck” or rate-limiting reaction immediately reduces metabolite flux through the entire pathway. Conversely, an increase in either its quantity or catalytic efficiency enhances flux through the pathway as a whole. For example, acetyl-CoA carboxylase catalyzes the synthesis of malonyl-CoA, the first committed reaction of fatty acid biosynthesis (Chapter 23). When synthesis of malonyl-CoA is inhibited, subsequent reactions of fatty acid synthesis cease for lack of substrates. As natural “governors” of metabolic flux, the enzymes that catalyze rate-limiting steps also constitute efficient targets for regulatory intervention by drugs. For example, “statin” drugs curtail synthesis of cholesterol by inhibiting HMG-CoA reductase, which catalyzes the rate-limiting reaction of cholesterogenesis.
REGULATION OF ENZYME QUANTITY
The catalytic capacity of the rate-limiting reaction in a metabolic pathway is the product of the concentration of enzyme molecules and their intrinsic catalytic efficiency. It therefore follows that catalytic capacity can be influenced both by changing the quantity of enzyme present and by altering its intrinsic catalytic efficiency.
Proteins Are Continuously Synthesized and Degraded
By measuring the rates of incorporation of 15N-labeled amino acids into protein and the rates of loss of 15N from protein, Schoenheimer deduced that body proteins are in a state of “dynamic equilibrium” in which they are continuously synthesized and degraded—a process referred to as protein turnover. This holds even for those proteins that are present at an essentially constant, or constitutive, steady-state level over time. On the other hand, the concentrations of many enzymes are influenced by a wide range of physiologic, hormonal, or dietary factors.
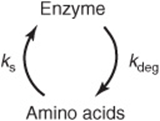
The absolute quantity of an enzyme reflects the net balance between its rate of synthesis and its rate of degradation. In human subjects, alterations in the levels of specific enzymes can be effected by a change in the rate constant for the overall processes of synthesis (ks), degradation (kdeg), or both.
Control of Enzyme Synthesis
The synthesis of certain enzymes depends upon the presence of inducers, typically substrates or structurally related compounds that stimulate the transcription of the gene that encodes them (Chapters 36 and 37). Escherichia coligrown on glucose will, for example, only catabolize lactose after addition of a β-galactoside, an inducer that triggers synthesis of a β-galactosidase and a galactoside permease (Figure 38–3). Inducible enzymes of humans include tryptophan pyrrolase, threonine dehydratase, tyrosine-α-ketoglutarate aminotransferase, enzymes of the urea cycle, HMG-CoA reductase, and cytochrome P450. Conversely, an excess of a metabolite may curtail synthesis of its cognate enzyme via repression. Both induction and repression involve cis elements, specific DNA sequences located upstream of regulated genes, and trans-acting regulatory proteins. The molecular mechanisms of induction and repression are discussed in Chapter 38. The synthesis of other enzymes can be stimulated by the interaction of hormones and other extracellular signals with specific cell-surface receptors. Detailed information on the control of protein synthesis in response to hormonal stimuli can be found in Chapter 42.
Control of Enzyme Degradation
In animals many proteins are degraded by the ubiquitin-proteasome pathway, the discovery of which earned Aaron Ciechanover, Avram Hershko, and Irwin Rose a Nobel Prize. Degradation takes place in the 26S proteasome, a large macromolecular complex made up of more than 30 polypeptide subunits arranged in the form of a hollow cylinder. The active sites of its proteolytic subunits face the interior of the cylinder, thus preventing indiscriminate degradation of cellular proteins. Proteins are targeted to the interior of the proteasome by “ubiquitination,” the covalent attachment of one or more ubiquitin molecules. Ubiquitin is a small, approximately 75 residue, protein that is highly conserved among eukaryotes. Ubiquitination is catalyzed by a large family of enzymes called E3 ligases, which attach ubiquitin to the side-chain amino group of lysyl residues.
The ubiquitin-proteasome pathway is responsible both for the regulated degradation of selected cellular proteins (for example, cyclins—Chapter 35) and for the removal of defective or aberrant protein species. The key to the versatility and selectivity of the ubiquitin-proteasome system resides in both the variety of intracellular E3 ligases and their ability to discriminate between the different physical or conformational states of target proteins. Thus, the ubiquitin-proteasome pathway can selectively degrade proteins whose physical integrity and functional competency have been compromised by the loss of or damage to a prosthetic group, oxidation of cysteine or histidine residues, or deamidation of asparagine or glutamine residues. Recognition by proteolytic enzymes also can be regulated by covalent modifications such as phosphorylation; binding of substrates or allosteric effectors; or association with membranes, oligonucleotides, or other proteins. A growing body of evidence suggests that dysfunctions of the ubiquitin-proteasome pathway contribute to the accumulation of aberrantly folded protein species characteristic of several neurodegenerative diseases.
MULTIPLE OPTIONS ARE AVAILABLE FOR REGULATING CATALYTIC ACTIVITY
In humans the induction of protein synthesis is a complex multistep process that typically requires hours to produce significant changes in overall enzyme level. By contrast, changes in intrinsic catalytic efficiency effected by binding of dissociable ligands (allosteric regulation) or by covalent modification achieve regulation of enzymic activity within seconds. Consequently, changes in protein level generally dominate when meeting long-term adaptive requirements, whereas changes in catalytic efficiency are best suited for rapid and transient alterations in metabolite flux.
ALLOSTERIC EFFECTORS REGULATE CERTAIN ENZYMES
Feedback inhibition refers to the process by which the end product of a multistep biosynthetic pathway binds to and inhibits an enzyme catalyzing one of the early steps in that pathway. In the following example, for the biosynthesis of D from A catalyzed by enzymes Enz1 through Enz3:
![]()
high concentrations of D inhibit the conversion of A to B. In this example, the feedback inhibitor D acts as a negative allosteric effector of Enz1. Inhibition results, not from the “backing up” of intermediates, but from the ability of D to bind to and inhibit Enz1. Generally, D binds at an allosteric site, one spatially distinct from the catalytic site of the target enzyme. Feedback inhibitors thus typically bear little or no structural similarity to the substrates of the enzymes they inhibit. For example, NAD+ and 3-phosphogylcerate, the substrates for 3-phosphgylcerate dehydrogenase, which catalyzes the first committed step in serine biosynthesis, bear no resemblance to the feedback inhibitor serine. In branched biosynthetic pathways, such as those responsible for nucleotide biosynthesis (Chapter 33), the initial reactions supply intermediates required for the synthesis of multiple end products. Figure 9–4 shows a hypothetical branched biosynthetic pathway in which curved arrows lead from feedback inhibitors to the enzymes whose activity they inhibit. The sequences S3 → A, S4 → B, S4 → C, and S3 → → D each represent linear reaction sequences that are feedback-inhibited by their end products. Branch point enzymes thus can be targeted to route metabolite flow.
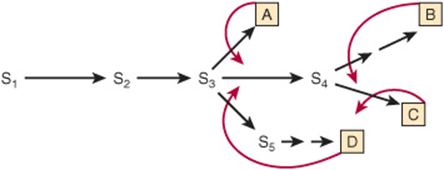
FIGURE 9–4 Sites of feedback inhibition in a branched biosynthetic pathway. S1–S5 are intermediates in the biosynthesis of end products A–D. Straight arrows represent enzymes catalyzing the indicated conversions. Curved red arrows represent feedback loops and indicate sites of feedback inhibition by specific end products.
Feedback inhibitors typically inhibit the first committed step in a particular biosynthetic sequence. The kinetics of feedback inhibition may be competitive, noncompetitive, partially competitive, or mixed. Layering multiple feedback loops can provide additional fine control. For example, as shown in Figure 9–5, the presence of excess product B decreases the requirement for substrate S2. However, S2 is also required for synthesis of A, C, and D. Therefore, for this pathway, excess B curtails synthesis of all four end products, regardless of the need for the other three. To circumvent this potential difficulty, each end product may only partially inhibit catalytic activity. The effect of an excess of two or more end products may be strictly additive or, alternatively, greater than their individual effect (cooperative feedback inhibition).
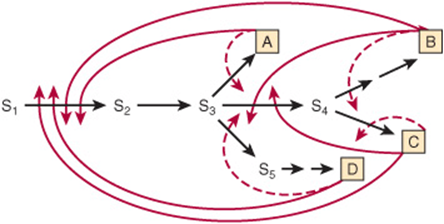
FIGURE 9–5 Multiple feedback inhibition in a branched biosynthetic pathway. Superimposed on simple feedback loops (dashed red arrows) are multiple feedback loops (solid red arrows) that regulate enzymes common to biosynthesis of several end products.
Aspartate Transcarbamoylase Is a Model Allosteric Enzyme
Aspartate transcarbamoylase (ATCase), the catalyst for the first reaction unique to pyrimidine biosynthesis (Figure 33–9), is a target of feedback regulation by two nucleotide triphosphates: cytidine triphosphate (CTP) and adenosine triphosphate. CTP, an end product of the pyrimidine biosynthetic pathway, inhibits ATCase, whereas the purine nucleotide ATP activates it. Moreover, high levels of ATP can overcome inhibition by CTP, enabling synthesis of pyrimidine nucleotides to proceed when purine nucleotide levels are elevated.
Allosteric & Catalytic Sites Are Spatially Distinct
Jacques Monod proposed the existence of allosteric sites that are physically distinct from the catalytic site. He reasoned that the lack of structural similarity between a feedback inhibitor and the substrate(s) for the enzyme whose activity it regulates indicated that these effectors are not isosteric with a substrate but allosteric (“occupy another space”). Allosteric enzymes thus are those for which catalysis at the active site may be modulated by the presence of effectors at an allosteric site. The existence of spatially distinct active and allosteric sites has since been verified in several enzymes using many lines of evidence. For example, x-ray crystallography revealed that the ATCase of E coli consists of six catalytic subunits and six regulatory subunits, the latter of which bind the nucleotide triphosphates that modulate activity. In general, binding of an allosteric regulator induces a conformational change in the enzyme that encompasses the active site.
Allosteric Effects May Be on Km or on Vmax
To refer to the kinetics of allosteric inhibition as “competitive” or “noncompetitive” with substrate carries misleading mechanistic implications. We refer instead to two classes of allosterically regulated enzymes: K-series and V-series enzymes. For K-series allosteric enzymes, the substrate saturation kinetics is competitive in the sense that Km is raised without an effect on Vmax. For V-series allosteric enzymes, the allosteric inhibitor lowers Vmax without affecting the Km. Alterations in Km or Vmax often are the product of conformational changes at the catalytic site induced by binding of the allosteric effector at its site. For a K-series allosteric enzyme, this conformational change may weaken the bonds between substrate and substrate-binding residues. For a V-series allosteric enzyme, the primary effect may be to alter the orientation or charge of catalytic residues, lowering Vmax. Intermediate effects on Kmand Vmax, however, may be observed consequent to these conformational changes.
FEEDBACK REGULATION IS NOT SYNONYMOUS WITH FEEDBACK INHIBITION
In both mammalian and bacterial cells, some end products “feed back” to control their own synthesis, in many instances by feedback inhibition of an early biosynthetic enzyme. We must, however, distinguish between feedback regulation, a phenomenologic term devoid of mechanistic implications, and feedback inhibition, a mechanism for regulation of enzyme activity. For example, while dietary cholesterol decreases hepatic synthesis of cholesterol, this feedback regulation does not involve feedback inhibition. HMG-CoA reductase, the rate-limiting enzyme of cholesterogenesis, is affected, but cholesterol does not inhibit its activity. Rather, regulation in response to dietary cholesterol involves curtailment by cholesterol or a cholesterol metabolite of the expression of the gene that encodes HMG-CoA reductase (enzyme repression) (Chapter 26).
MANY HORMONES ACT THROUGH ALLOSTERIC SECOND MESSENGERS
Nerve impulses and the binding of many hormones to cell surface receptors elicit changes in the rate of enzyme-catalyzed reactions within target cells by inducing the release or synthesis of specialized allosteric effectors called second messengers. The primary, or “first,” messenger is the hormone molecule or nerve impulse. Second messengers include 3′, 5′-cAMP, synthesized from ATP by the enzyme adenylyl cyclase in response to the hormone epinephrine, and Ca2+, which is stored inside the endoplasmic reticulum of most cells. Membrane depolarization resulting from a nerve impulse opens a membrane channel that releases calcium ions into the cytoplasm, where they bind to and activate enzymes involved in the regulation of muscle contraction and the mobilization of stored glucose from glycogen. Glucose then supplies the increased energy demands of muscle contraction. Other second messengers include 3′,5′-cGMP, nitric oxide, and the polyphosphoinositols produced by the hydrolysis of inositol phospholipids by hormone-regulated phospholipases. Specific examples of the participation of second messengers in the regulation of cellular processes can be found in Chapters 19, 42, and 48.
REGULATORY COVALENT MODIFICATIONS CAN BE REVERSIBLE OR IRREVERSIBLE
In mammalian cells, a wide range of regulatory covalent modifications occur. Partial proteolysis and phosphorylation, for
example, are frequently employed to regulate the catalytic activity of enzymes. On the other hand, histones and other DNA binding proteins in chromatin are subject to extensive modification by acetylation, methylation, ADP-ribosylation, as well as phosphorylation. The latter modifications, which modulate the manner in which the proteins within chromatin interact with each other as well as the DNA itself, constitute the basis for the “histone code.” The resulting changes in chromatin structure within the region affected can render genes more accessible to the protein responsible for their transcription, thereby enhancing gene expression or, on a larger scale, facilitating replication of the entire genome (Chapter 38). On the other hand, changes in chromatin structure that restrict the accessibility of genes to transcription factors, DNA-dependent RNA polymerases, etc, thereby inhibiting transcription, are said to silence gene expression.
The histone code represents a classic example of epigenetics, the hereditary transmission of information by a means other than the sequence of nucleotides that comprise the genome. In this instance, the pattern of gene expression within a newly formed “daughter” cell will be determined, in part, by the particular set of histone covalent modifications embodied in the chromatin proteins inherited from the “parental” cell.
Acetylation, ADP-ribosylation, methylation, and phosphorylation are all examples of “reversible” covalent modifications. In this instance, reversible refers to the fact that the modified protein can be restored to its original, modification-free state. It does not, however, refer to the mechanisms by which such restoration takes place. Thermodynamics dictates that if the enzyme-catalyzed reaction by which the modification was introduced is thermodynamically favorable, the free energy change involved in simply trying to run the reaction in reverse will be unfavorable. The phosphorylation of proteins on seryl, threonyl, or tyrosyl residues, catalyzed by protein kinases, is thermodynamically favored as a consequence of utilizing the high-energy gamma phosphoryl group of ATP. Phosphate groups are removed, not by recombining the phosphate with ADP to form ATP, but by a hydrolytic reaction catalyzed by enzymes called protein phosphatases. Similarly, acetyltransferases employ a high-energy donor substrate, NAD+, while deacetylases catalyze a direct hydrolysis that generates free acetate.
Because the high entropic barrier prevents the reunification of the two portions of a protein produced by hydrolysis of a peptide bond, proteolysis constitutes a physiologically irreversible modification. Once a proprotein is activated, it will continue to carry out its catalytic or other functions until it is removed by degradation or some other means. Zymogen activation thus represents a simple and economical, albeit one way, mechanism for restraining the latent activity of a protein until the appropriate circumstances are encountered. It is therefore not surprising that partial proteolysis is employed frequently to regulate proteins that work in the gastrointestinal tract or bloodstream rather than in the interior of cells.
PROTEASES MAY BE SECRETED AS CATALYTICALLY INACTIVE PROENZYMES
Certain proteins are synthesized and secreted as inactive precursor proteins known as proproteins. Selective, or “partial,” proteolysis converts a proprotein by one or more successive proteolytic “clips” to a form that exhibits the characteristic activity of the mature protein, for example, its catalytic activity. The proprotein forms of enzymes are termed proenzymes or zymogens. Proteins synthesized as proproteins include the hormone insulin (proprotein = proinsulin), the digestive enzymes pepsin, trypsin, and chymotrypsin (proproteins = pepsinogen, trypsinogen, and chymotrypsinogen, respectively), several factors of the blood clotting and blood clot dissolution cascades (see Chapter 51), and the connective tissue protein collagen (proprotein = procollagen).
Proenzymes Facilitate Rapid Mobilization of an Activity in Response to Physiologic Demand
The synthesis and secretion of proteases as catalytically inactive proenzymes protect the tissue of origin (eg, the pancreas) from autodigestion, such as can occur in pancreatitis. Certain physiologic processes such as digestion are intermittent but fairly regular and predictable in frequency. Others such as blood clot formation, clot dissolution, and tissue repair are brought “on line” only in response to pressing physiologic or pathophysiologic need. The processes of blood clot formation and dissolution clearly must be temporally coordinated to achieve homeostasis. Enzymes needed intermittently but rapidly often are secreted in an initially inactive form since new synthesis and secretion of the required proteins might be insufficiently rapid to respond to a pressing pathophysiologic demand such as the loss of blood (see Chapter 51).
Activation of Prochymotrypsin Requires Selective Proteolysis
Selective proteolysis involves one or more highly specific proteolytic clips that may or may not be accompanied by separation of the resulting peptides. Most importantly, selective proteolysis often results in conformational changes that “create” the catalytic site of an enzyme. Note that while the catalytically essential residues His 57 and Asp 102 reside on the B peptide of α-chymotrypsin, Ser 195 resides on the C peptide (Figure 9–6). The conformational changes that accompany selective proteolysis of prochymotrypsin (chymotrypsinogen) align the three residues of the charge-relay network (see Figure 7–7), forming the catalytic site. Note also that contact and catalytic residues can be located on different peptide chains but still be within bond-forming distance of bound substrate.
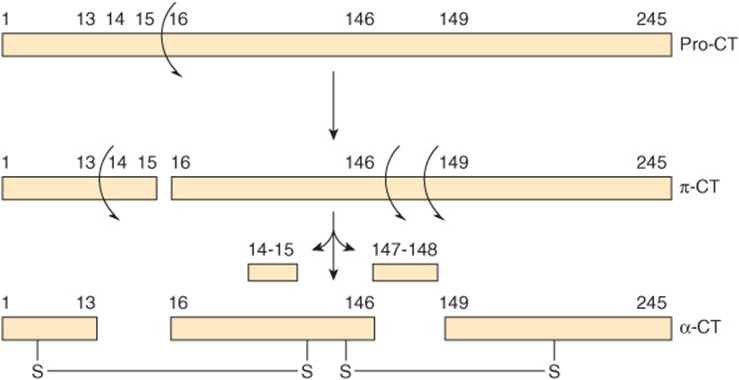
FIGURE 9–6 Two-dimensional representation of the sequence of proteolytic events that ultimately result in formation of the catalytic site of chymotrypsin, which includes the Asp 102-His57-Ser195 catalytic triad (see Figure 7–7). Successive proteolysis forms prochymotrypsin (pro-CT), π-chymotrypsin (π-Ct), and ultimately α-chymotrypsin (α-CT), an active protease whose three peptides (A, B, C) remain associated by covalent inter-chain disulfide bonds.
REVERSIBLE COVALENT MODIFICATION REGULATES KEY MAMMALIAN PROTEINS
Mammalian proteins are the targets of a wide range of covalent modification processes. Modifications such as prenylation, glycosylation, hydroxylation, and fatty acid acylation introduce unique structural features into newly synthesized proteins that tend to persist for the lifetime of the protein. Among the covalent modifications that regulate protein function (eg, methylation, acetylation), the most common by far is phosphorylation–dephosphorylation. Protein kinases phosphorylate proteins by catalyzing transfer of the terminal phosphoryl group of ATP to the hydroxyl groups of seryl, threonyl, or tyrosyl residues, forming O-phosphoseryl, O-phosphothreonyl, or O-phosphotyrosyl residues, respectively (Figure 9–7). Some protein kinases target the side chains of histidyl, lysyl, arginyl, and aspartyl residues. The unmodified form of the protein can be regenerated by hydrolytic removal of phosphoryl groups, catalyzed by protein phosphatases.
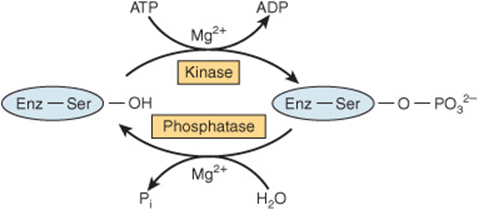
FIGURE 9–7 Covalent modification of a regulated enzyme by phosphorylation–dephosphorylation of a seryl residue.
A typical mammalian cell possesses thousands of phosphorylated proteins and several hundred protein kinases and protein phosphatases that catalyze their interconversion. The ease of interconversion of enzymes between their phospho-and dephospho- forms accounts, in part, for the frequency with which phosphorylation–dephosphorylation is utilized as a mechanism for regulatory control. Phosphorylation–dephosphorylation permits the functional properties of the affected enzyme to be altered only for as long as it serves a specific need. Once the need has passed, the enzyme can be converted back to its original form, poised to respond to the next stimulatory event. A second factor underlying the widespread use of protein phosphorylation–dephosphorylation lies in the chemical properties of the phosphoryl group itself. In order to alter an enzyme’s functional properties, any modification of its chemical structure must influence the protein’s three-dimensional configuration. The high charge density of protein-bound phosphoryl groups—generally –2 at physiologic pH—and their propensity to form strong salt bridges with arginyl and lysyl residues renders them potent agents for modifying protein structure and function. Phosphorylation generally influences an enzyme’s intrinsic catalytic efficiency or other properties by inducing conformational changes. Consequently, the amino acids targeted by phosphorylation can be and typically are relatively distant from the catalytic site itself.
Covalent Modifications Regulate Metabolite Flow
In many respects, sites of protein phosphorylation and other covalent modifications can be considered another form of allosteric site. However, in this case, the “allosteric ligand” binds covalently to the protein. Both phosphorylation-dephosphorylation and feedback inhibition provide short-term, readily reversible regulation of metabolite flow in response to specific physiologic signals. Both act without altering gene expression. Both act on early enzymes of a protracted biosynthetic metabolic pathway, and both act at allosteric rather than catalytic sites. Feedback inhibition, however, involves a single protein and lacks hormonal and neural features. By contrast, regulation of mammalian enzymes by phosphorylation–dephosphorylation involves several proteins and ATP, and is under direct neural and hormonal control.
PROTEIN PHOSPHORYLATION IS EXTREMELY VERSATILE
Protein phosphorylation–dephosphorylation is a highly versatile and selective process. Not all proteins are subject to phosphorylation, and of the many hydroxyl groups on a protein’s surface, only one or a small subset are targeted. While the most common enzyme function affected is the protein’s catalytic efficiency, phosphorylation can also alter its location within the cell, susceptibility to proteolytic degradation, or responsiveness to regulation by allosteric ligands. Phosphorylation can increase an enzyme’s catalytic efficiency, converting it to its active form in one protein, while phosphorylation of another protein converts it to an intrinsically inefficient, or inactive, form (Table 9–1).
TABLE 9–1 Examples of Mammalian Enzymes Whose Catalytic Activity Is Altered by Covalent Phosphorylation-Dephosphorylation
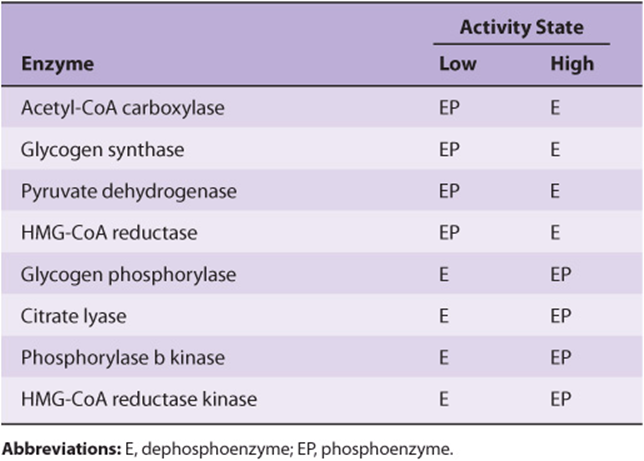
Many proteins can be phosphorylated at multiple sites. Others are subject to regulation both by phosphorylation–dephosphorylation and by the binding of allosteric ligands, or by phosphorylation–dephosphorylation and another covalent modification. Phosphorylation–dephosphorylation at any one site can be catalyzed by multiple protein kinases or protein phosphatases. Many protein kinases and most protein phosphatases act on more than one protein and are themselves interconverted between active and inactive forms by the binding of second messengers or by covalent modification by phosphorylation–dephosphorylation.
The interplay between protein kinases and protein phosphatases, between the functional consequences of phosphorylation at different sites, between phosphorylation sites and allosteric sites, or between phosphorylation sites and other sites of covalent modification provides the basis for regulatory networks that integrate multiple environmental input signals to evoke an appropriate coordinated cellular response. In these sophisticated regulatory networks, individual enzymes respond to different environmental signals. For example, if an enzyme can be phosphorylated at a single site by more than one protein kinase, it can be converted from a catalytically efficient to an inefficient (inactive) form, or vice versa, in response to any one of several signals. If the protein kinase is activated in response to a signal different from the signal that activates the protein phosphatase, the phosphoprotein becomes a decision node. The functional output, generally catalytic activity, reflects the phosphorylation state. This state or degree of phosphorylation is determined by the relative activities of the protein kinase and protein phosphatase, a reflection of the presence and relative strength of the environmental signals that act through each.
The ability of many protein kinases and protein phosphatases to target more than one protein provides a means for an environmental signal to coordinately regulate multiple metabolic processes. For example, the enzymes 3-hydroxy-3-methylglutaryl-CoA reductase and acetyl-CoA carboxylase—the rate-controlling enzymes for cholesterol and fatty acid biosynthesis, respectively—are phosphorylated and inactivated by the AMP-activated protein kinase. When this protein kinase is activated either through phosphorylation by yet another protein kinase or in response to the binding of its allosteric activator 5′-AMP, the two major pathways responsible for the synthesis of lipids from acetyl-CoA are both inhibited.
INDIVIDUAL REGULATORY EVENTS COMBINE TO FORM SOPHISTICATED CONTROL NETWORKS
Cells carry out a complex array of metabolic processes that must be regulated in response to a broad spectrum of environmental factors. Hence, interconvertible enzymes and the enzymes responsible for their interconvesion do not act as isolated “on” and “off” switches. In order to meet the demands of maintaining homeostasis, these building blocks are linked to form integrated regulatory networks.
One well-studied example of such a network is the eukaryotic cell cycle that controls cell division. Upon emergence from the G0 or quiescent state, the extremely complex process of cell division proceeds through a series of specific phases designated G1, S, G2, and M (Figure 9–8). Elaborate monitoring systems, called checkpoints, assess key indicators of progress to ensure that no phase of the cycle is initiated until the prior phase is complete. Figure 9–8 outlines, in simplified form, part of the checkpoint that controls the initiation of DNA replication, called the S phase. A protein kinase called ATM is associated with the genome. If the DNA contains a double-stranded break, the resulting change in the conformation of the chromatin activates ATM. Upon activation, one subunit of the activated ATM dimer dissociates and initiates a series, or cascade, of protein phosphorylation–dephosphorylation events mediated by the CHK1 and CHK2 protein kinases, the Cdc25 protein phosphatase, and finally a complex between a cyclin and a cyclin-dependent protein kinase, or Cdk. Activation of the Cdk-cyclin complex blocks the G1 to S transition, thus preventing the replication of damaged DNA. Failure at this checkpoint can lead to mutations in DNA that may lead to cancer or other diseases. Each step in the cascade provides a conduit for monitoring additional indicators of cell status prior to entering S phase.

FIGURE 9–8 A simplified representation of the G1 to S checkpoint of the eukaryotic cell cycle. The circle shows the various stages in the eukaryotic cell cycle. The genome is replicated during S phase, while the two copies of the genome are segregated and cell division occurs during M phase. Each of these phases is separated by a G, or growth, phase characterized by an increase in cell size and the accumulation of the precursors required for the assembly of the large macromolecular complexes formed during S and M phases.
SUMMARY
![]() Homeostasis involves maintaining a relatively constant intracellular and intra-organ environment despite wide fluctuations in the external environment. This is achieved via appropriate changes in the rates of biochemical reactions in response to physiologic need.
Homeostasis involves maintaining a relatively constant intracellular and intra-organ environment despite wide fluctuations in the external environment. This is achieved via appropriate changes in the rates of biochemical reactions in response to physiologic need.
![]() The substrates for most enzymes are usually present at a concentration close to their Km. This facilitates passive control of the rates of product formation in response to changes in levels of metabolic intermediates.
The substrates for most enzymes are usually present at a concentration close to their Km. This facilitates passive control of the rates of product formation in response to changes in levels of metabolic intermediates.
![]() Active control of metabolite flux involves changes in the concentration, catalytic activity, or both of an enzyme that catalyzes a committed, rate-limiting reaction.
Active control of metabolite flux involves changes in the concentration, catalytic activity, or both of an enzyme that catalyzes a committed, rate-limiting reaction.
![]() Selective proteolysis of catalytically inactive proenzymes initiates conformational changes that form the active site. Secretion as an inactive proenzyme facilitates rapid mobilization of activity in response to injury or physiologic need and may protect the tissue of origin (eg, autodigestion by proteases).
Selective proteolysis of catalytically inactive proenzymes initiates conformational changes that form the active site. Secretion as an inactive proenzyme facilitates rapid mobilization of activity in response to injury or physiologic need and may protect the tissue of origin (eg, autodigestion by proteases).
![]() Binding of metabolites and second messengers to sites distinct from the catalytic site of enzymes triggers conformational changes that alter Vmax or Km.
Binding of metabolites and second messengers to sites distinct from the catalytic site of enzymes triggers conformational changes that alter Vmax or Km.
![]() Phosphorylation by protein kinases of specific seryl, threonyl, or tyrosyl residues—and subsequent dephosphorylation by protein phosphatases—regulates the activity of many human enzymes. The protein kinases and phosphatases that participate in regulatory cascades that respond to hormonal or second messenger signals constitute regulatory networks that can process and integrate complex environmental information to produce an appropriate and comprehensive cellular response.
Phosphorylation by protein kinases of specific seryl, threonyl, or tyrosyl residues—and subsequent dephosphorylation by protein phosphatases—regulates the activity of many human enzymes. The protein kinases and phosphatases that participate in regulatory cascades that respond to hormonal or second messenger signals constitute regulatory networks that can process and integrate complex environmental information to produce an appropriate and comprehensive cellular response.
REFERENCES
Ciechanover A, Schwartz AL: The ubiquitin system: pathogenesis of human diseases and drug targeting. Biochim Biophys Acta 2004;1695:3.
Elgin SC, Reuter G. In Allis CD, Jenuwein T, Reinberg D, et al (editors): Epigenetics, Cold Spring Harbor Laboratory Press, 2007.
Johnson LN, Lewis RJ: Structural basis for control by phosphorylation. Chem Rev 2001;101:2209.
Muoio DM, Newgard CB: Obseity-related derangements in metabolic regulation. Anu Rev Biochem 2006;75:403.
Scriver CR, Sly WS, Childs B, et al (editors): The Metabolic and Molecular Bases of Inherited Disease, 8th ed. McGraw-Hill, 2000.
Stieglitz K, Stec B, Baker DP, et al: Monitoring the transition from the T to the R state in E. coli aspartate transcarbamoylase by x-ray crystallography: crystal structures of the E50A mutant enzyme in four distinct allosteric states. J Mol Biol 2004;341:853.
Tu BP, Kudlicki A, Rowicka M, et al: Logic of the yeast metabolic cycle: temporal compartmentalization of cellular processes. Science 2005;310:1152.
Walsh CT: Posttranslational Modification of Proteins. Expanding Nature’s Inventory, Roberts and Company Publishers, 2006.