Harper’s Illustrated Biochemistry, 29th Edition (2012)
SECTION II. Bioenergetics & the Metabolism of Carbohydrates & Lipids
Chapter 13. The Respiratory Chain & Oxidative Phosphorylation
Kathleen M. Botham, PhD, DSc & Peter A. Mayes, PhD, DSc
OBJECTIVES
After studying this chapter, you should be able to:
![]() Describe the double membrane structure of mitochondria and indicate the location of various enzymes.
Describe the double membrane structure of mitochondria and indicate the location of various enzymes.
![]() Appreciate that energy from the oxidation of fuel substrates (fats, carbohydrates, amino acids) is almost all liberated in mitochondria as reducing equivalents, which are passed by a process termed electron transport through a series of redox carriers or complexes embedded in the inner mitochondrial membrane known as the respiratory chain until they are finally reacted with oxygen to form water.
Appreciate that energy from the oxidation of fuel substrates (fats, carbohydrates, amino acids) is almost all liberated in mitochondria as reducing equivalents, which are passed by a process termed electron transport through a series of redox carriers or complexes embedded in the inner mitochondrial membrane known as the respiratory chain until they are finally reacted with oxygen to form water.
![]() Describe the four protein complexes involved in the transfer of electrons through the respiratory chain and explain the roles of flavoproteins, iron sulfur proteins, and coenzyme Q.
Describe the four protein complexes involved in the transfer of electrons through the respiratory chain and explain the roles of flavoproteins, iron sulfur proteins, and coenzyme Q.
![]() Understand how coenzyme Q accepts electrons from NADH via Complex I and from FADH2 via Complex II.
Understand how coenzyme Q accepts electrons from NADH via Complex I and from FADH2 via Complex II.
![]() Indicate how electrons are passed from reduced coenzyme Q to cytochrome c via Complex III in the Q cycle.
Indicate how electrons are passed from reduced coenzyme Q to cytochrome c via Complex III in the Q cycle.
![]() Explain the process by which reduced cytochrome c is oxidized and oxygen is reduced to water via Complex IV.
Explain the process by which reduced cytochrome c is oxidized and oxygen is reduced to water via Complex IV.
![]() Understand how electron transport through the respiratory chain generates a proton gradient across the inner mitochondrial membrane, leading to the buildup of a proton motive force that generates ATP by the process of oxidative phosphorylation.
Understand how electron transport through the respiratory chain generates a proton gradient across the inner mitochondrial membrane, leading to the buildup of a proton motive force that generates ATP by the process of oxidative phosphorylation.
![]() Describe the structure of the ATP synthase enzyme and explain how it works as a rotary motor to produce ATP from ADP and Pi.
Describe the structure of the ATP synthase enzyme and explain how it works as a rotary motor to produce ATP from ADP and Pi.
![]() Identify the five conditions controlling the rate of respiration in mitochondria and understand that oxidation of reducing equivalents via the respiratory chain and oxidative phosphorylation are tightly coupled in most circumstances, so that one cannot proceed unless the other is functioning.
Identify the five conditions controlling the rate of respiration in mitochondria and understand that oxidation of reducing equivalents via the respiratory chain and oxidative phosphorylation are tightly coupled in most circumstances, so that one cannot proceed unless the other is functioning.
![]() Indicate examples of common poisons that block respiration or oxidative phosphorylation and identify their site of action.
Indicate examples of common poisons that block respiration or oxidative phosphorylation and identify their site of action.
![]() Explain, with examples, how uncouplers may act as poisons by dissociating oxidation via the respiratory chain from oxidative phosphorylation, but may also have a physiological role in generating body heat.
Explain, with examples, how uncouplers may act as poisons by dissociating oxidation via the respiratory chain from oxidative phosphorylation, but may also have a physiological role in generating body heat.
![]() Explain the role of exchange transporters present in the inner mitochondrial membrane in allowing ions and metabolites to pass through while preserving electrochemical and osmotic equilibrium.
Explain the role of exchange transporters present in the inner mitochondrial membrane in allowing ions and metabolites to pass through while preserving electrochemical and osmotic equilibrium.
BIOMEDICAL IMPORTANCE
Aerobic organisms are able to capture a far greater proportion of the available free energy of respiratory substrates than anaerobic organisms. Most of this takes place inside mitochondria, which have been termed the “powerhouses” of the cell. Respiration is coupled to the generation of the high-energy intermediate, ATP, by oxidative phosphorylation. A number of drugs (eg, amobarbital) and poisons (eg, cyanide, carbon monoxide) inhibit oxidative phosphorylation, usually with fatal consequences. Several inherited defects of mitochondria involving components of the respiratory chain and oxidative phosphorylation have been reported. Patients present with myopathy and encephalopathy and often have lactic acidosis.
SPECIFIC ENZYMES ACT AS MARKERS OF COMPARTMENTS SEPARATED BY THE MITOCHONDRIAL MEMBRANES
Mitochondria have an outer membrane that is permeable to most metabolites and an inner membrane that is selectively permeable, enclosing a matrix within (Figure 13–1). The outer membrane is characterized by the presence of various enzymes, including acyl-CoA synthetase and glycerophosphate acyltransferase. Adenylyl kinase and creatine kinase are found in the intermembrane space. The phospholipid cardiolipin is concentrated in the inner membrane together with the enzymes of the respiratory chain, ATP synthase, and various membrane transporters.
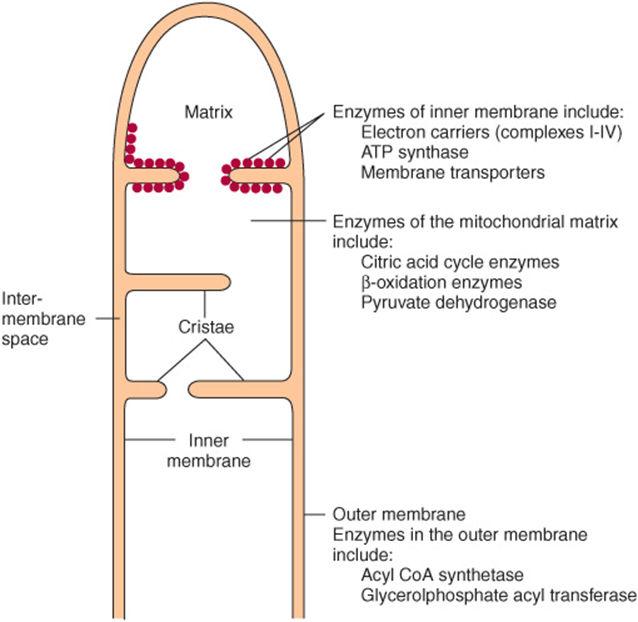
FIGURE 13–1 Structure of the mitochondrial membranes. Note that the inner membrane contains many folds or cristae.
THE RESPIRATORY CHAIN OXIDIZES REDUCING EQUIVALENTS & ACTS AS A PROTON PUMP
Most of the energy liberated during the oxidation of carbohydrate, fatty acids, and amino acids is made available within mitochondria as reducing equivalents (—H or electrons) (Figure 13–2). Note that the enzymes of the citric acid cycle and β-oxidation (Chapters 22 & 17) are contained in mitochondria, together with the respiratory chain, which collects and transports reducing equivalents, directing them to their final reaction with oxygen to form water, and the machinery for oxidative phosphorylation, the process by which the liberated free energy is trapped as high-energy phosphate.
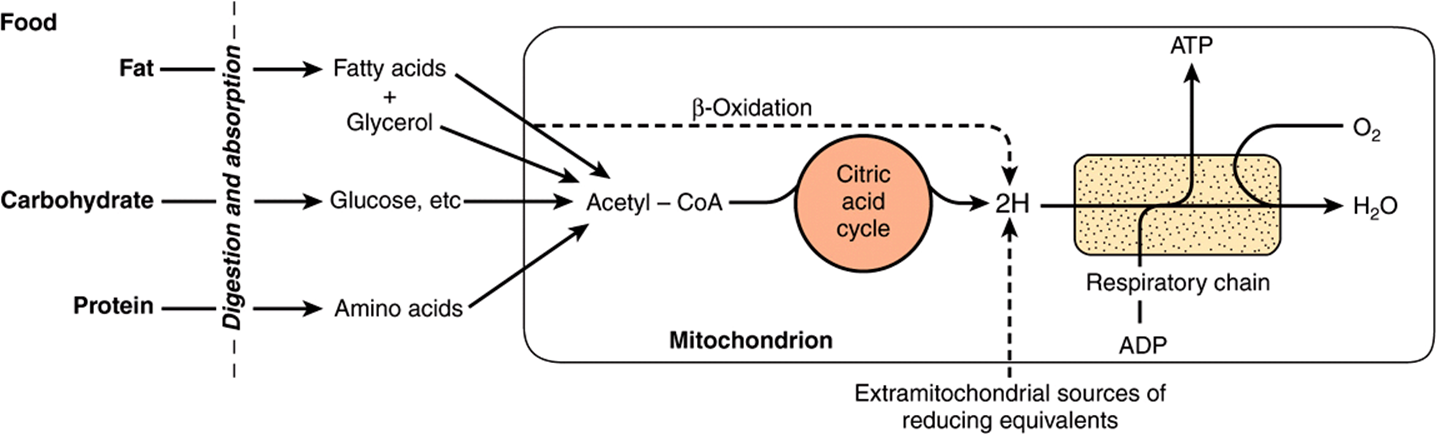
FIGURE 13–2 Role of the respiratory chain of mitochondria in the conversion of food energy to ATP. Oxidation of the major foodstuffs leads to the generation of reducing equivalents (2H) that are collected by the respiratory chain for oxidation and coupled generation of ATP.
Components of the Respiratory Chain Are Contained in Four Large Protein Complexes Embedded in the Inner Mitochondrial Membrane
Electrons flow through the respiratory chain through a redox span of 1.1 V from NAD+/NADH to O2/2H2O (Table 12-1), passing through three large protein complexes: NADH-Q oxidoreductase (Complex I), where electrons are transferred from NADH to coenzyme Q (Q) (also called ubiquinone); Q-cytochrome c oxidoreductase (Complex III), which passes the electrons on to cytochrome c; and cytochrome c oxidase (Complex IV), which completes the chain, passing the electrons to O2 and causing it to be reduced to H2O (Figure 13–3). Some substrates with more positive redox potentials than NAD+/NADH (eg, succinate) pass electrons to Q via a fourth complex, succinate-Q reductase (Complex II), rather than Complex I. The four complexes are embedded in the inner mitochondrial membrane, but Q and cytochrome c are mobile. Q diffuses rapidly within the membrane, while cytochrome c is a soluble protein. The flow of electrons through Complexes I, III, and IV results in the pumping of protons from the matrix across the inner mitochondrial membrane into the intermembrane space (Figure 13–7).
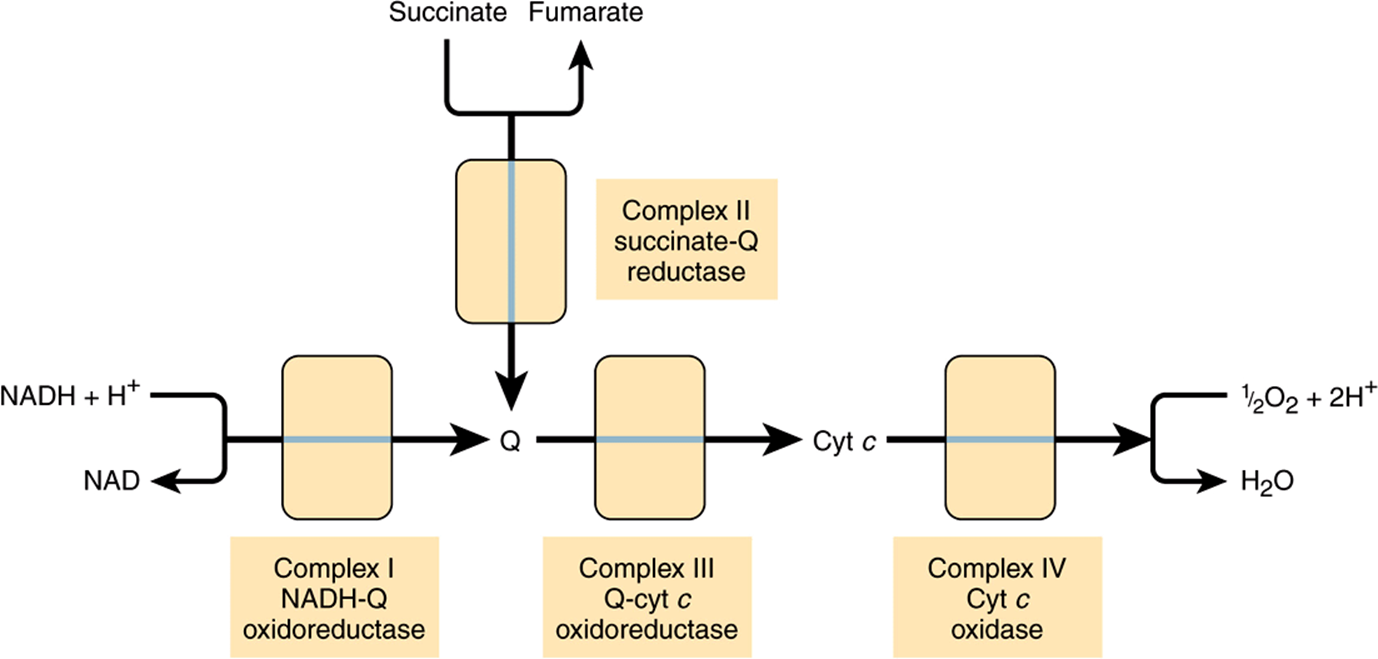
FIGURE 13–3 Overview of electron flow through the respiratory chain. (cyt, cytochrome; Q, coenzyme Q or ubiquinone.)
Flavoproteins and Iron-Sulfur Proteins (Fe-S) Are Components of the Respiratory Chain Complexes
Flavoproteins (Chapter 12) are important components of Complexes I and II. The oxidized flavin nucleotide (FMN or FAD) can be reduced in reactions involving the transfer of two electrons (to form FMNH2 or FADH2), but they can also accept one electron to form the semiquinone (Figure 12–2). Iron-sulfur proteins (nonheme iron proteins, Fe-S) are found in Complexes I, II, and III. These may contain one, two, or four Fe atoms linked to inorganic sulfur atoms and/or via cysteine-SH groups to the protein (Figure 13–4). The Fe-S take part in single electron transfer reactions in which one Fe atom undergoes oxidoreduction between Fe2+ and Fe3+.
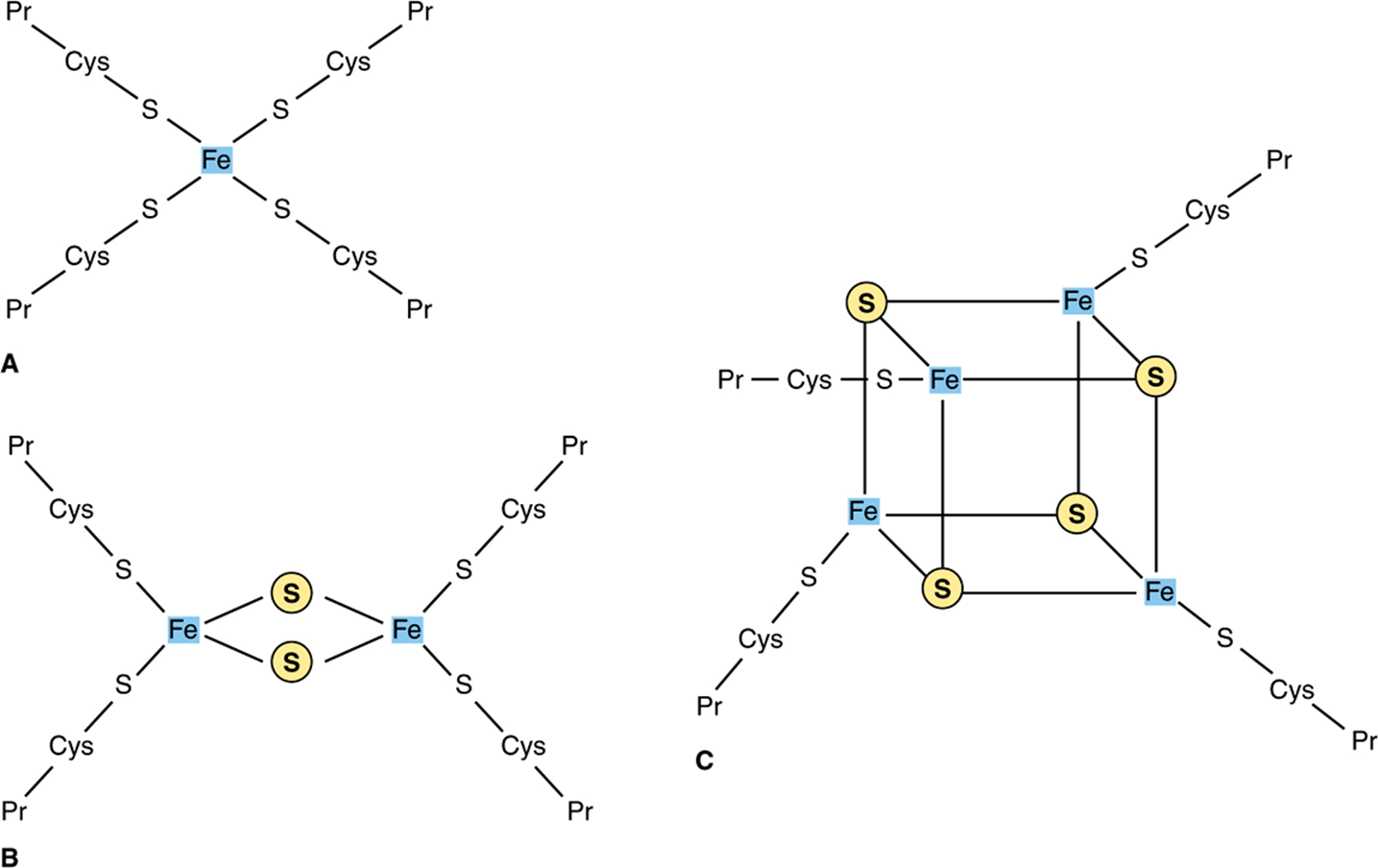
FIGURE 13–4 Iron-sulfur proteins (Fe-S). (A) The simplest Fe-S with one Fe bound by fourcysteines. (B) 2Fe-2S center. (C) 4Fe-4S center. (Cys, cysteine; Pr, apoprotein; ![]() , Inorganic sulfur.)
, Inorganic sulfur.)
Q Accepts Electrons via Complexes I and II
NADH-Q oxidoreductase or Complex I is a large L-shaped multisubunit protein that catalyzes electron transfer from NADH to Q, coupled with the transfer of four H+ across the membrane:
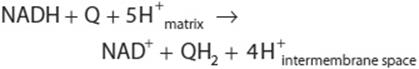
Electrons are transferred from NADH to FMN initially, then to a series of Fe-S centers, and finally to Q (Figure 13–5). In Complex II (succinate-Q reductase), FADH2 is formed during the conversion of succinate to fumarate in the citric acid cycle (Figure 17–3) and electrons are then passed via several Fe-S centers to Q (Figure 13–5). Glycerol-3-phosphate (generated in the breakdown of triacylglycerols or from glycolysis, Figure 18–2) and acyl-CoA also pass electrons to Q via different pathways involving flavoproteins (Figure 13–5).
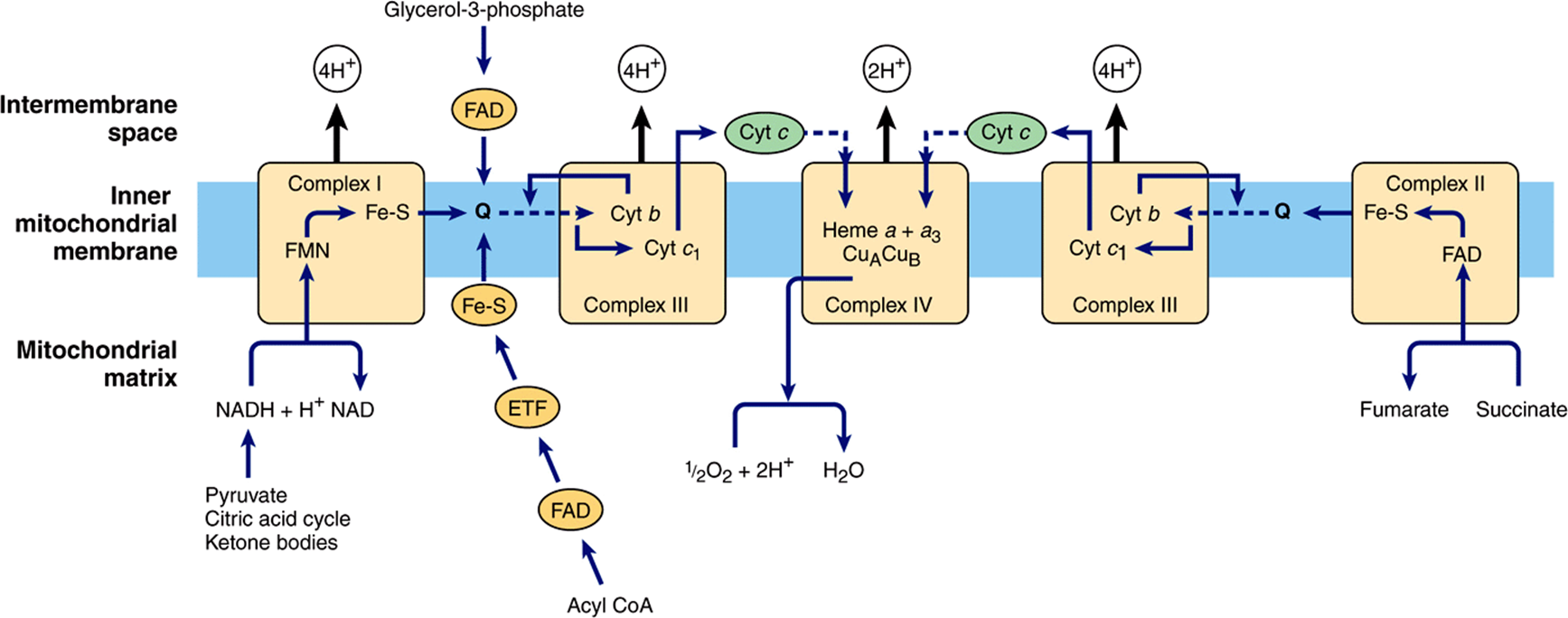
FIGURE 13–5 Flow of electrons through the respiratory chain complexes, showing the entry points for reducing equivalents from important substrates. Q and cyt c are mobile components of the system as indicated by the dotted arrows. The flow through Complex III (the Q cycle) is shown in more detail in Figure 13–6. (ETF, electron transferring flavoprotein; Fe-S, iron-sulfur protein; cyt, cytochrome; Q, coenzyme Q or ubiquinone.)
The Q Cycle Couples Electron Transfer to Proton Transport in Complex III
Electrons are passed from QH2 to cytochrome c via Complex III (Q-cytochrome c oxidoreductase):
![]()
The process is believed to involve cytochromes c1, bL and bH and a Rieske Fe-S (an unusual Fe-S in which one of the Fe atoms is linked to two histidine residues rather than two cysteine residues) (Figure 13–5) and is known as the Q cycle (Figure 13–6). Q may exist in three forms: the oxidized quinone, the reduced quinol, or the semiquinone (Figure 13–6). The semiquinone is formed transiently during the cycle, one turn of which results in the oxidation of 2QH2 to Q, releasing 4H+ into the intermembrane space, and the reduction of one Q to QH2, causing 2H+ to be taken up from the matrix (Figure 13–6). Note that while Q carries two electrons, the cytochromes carry only one, thus the oxidation of one QH2 is coupled to the reduction of two molecules of cytochrome c via the Q cycle.
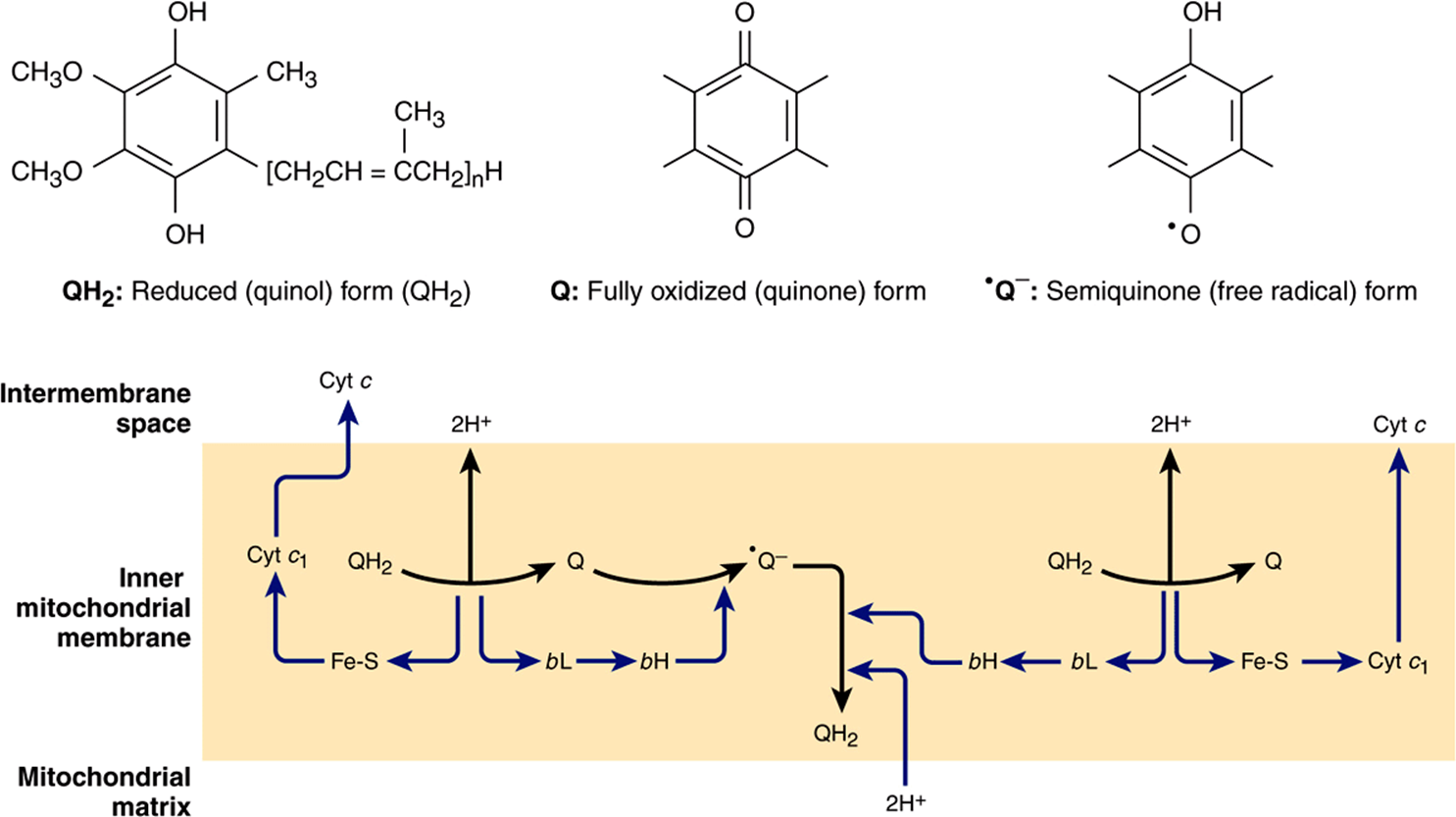
FIGURE 13–6 The Q cycle. During the oxidation of QH2 to Q, one electron is donated to cyt c via a Rieske Fe-S and cyt c1 and the second to a Q to formthe semiquinone via cyt bL and cyt bH, with 2H+ being released into the intermembrane space. A similar process then occurs with a second QH2, but in this case the second electron is donated to the semiquinone, reducing it to QH2, and 2H+ are taken up from the matrix. (cyt, cytochrome; Fe-S, iron-sulfur protein; Q, coenzyme Q or ubiquinone.)
Molecular Oxygen Is Reduced to Water via Complex IV
Reduced cytochrome c is oxidized by Complex IV (cytochrome c oxidase), with the concomitant reduction of O2 to two molecules of water:
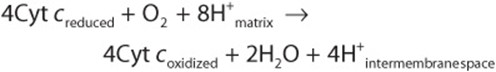
This transfer of four electrons from cytochrome c to O2 involves two heme groups, a and a3, and Cu (Figure 13–5). Electrons are passed initially to a Cu center (CuA), which contains 2Cu atoms linked to two protein cysteine-SH groups (resembling an Fe-S), then in sequence to heme a, heme a3, a second Cu center, CuB, which is linked to heme a3, and finally to O2. Of the eight H+ removed from the matrix, four are used to form two water molecules and four are pumped into the intermembrane space. Thus, for every pair of electrons passing down the chain from NADH or FADH2, 2H+ are pumped across the membrane by Complex IV. The O2 remains tightly bound to Complex IV until it is fully reduced, and this minimizes the release of potentially damaging intermediates such as superoxide anions or peroxide which are formed when O2 accepts one or two electrons, respectively (Chapter 12).
ELECTRON TRANSPORT VIA THE RESPIRATORY CHAIN CREATES A PROTON GRADIENT WHICH DRIVES THE SYNTHESIS OF ATP
The flow of electrons through the respiratory chain generates ATP by the process of oxidative phosphorylation. The che-miosmotic theory, proposed by Peter Mitchell in 1961, postulates that the two processes are coupled by a proton gradient across the inner mitochondrial membrane so that the proton motive force caused by the electrochemical potential difference (negative on the matrix side) drives the mechanism of ATP synthesis. As we have seen, Complexes I, III, and IV act as proton pumps. Since the inner mitochondrial membrane is impermeable to ions in general and particularly to protons, these accumulate in the intermembrane space, creating the proton motive force predicted by the chemiosmotic theory.
A Membrane-Located ATP Synthase Functions as a Rotary Motor to Form ATP
The proton motive force drives a membrane-located ATP synthase that forms ATP in the presence of Pi + ADP. ATP synthase is embedded in the inner membrane, together with the respiratory chain complexes (Figure 13–7).Several subunits of the protein form a ball-like shape arranged around an axis known as F1, which projects into the matrix and contains the phosphorylation mechanism (Figure 13–8). F1 is attached to a membrane protein complex known as F0, which also consists of several protein subunits. F0 spans the membrane and forms a proton channel. The flow of protons through F0 causes it to rotate, driving the production of ATP in the F1 complex (Figures 13-7 and 13-8). This is thought to occur by a binding change mechanism in which the conformation of the β-subunits in F1 is changed as the axis rotates from one that binds ATP tightly to one that releases ATP and binds ADP and Pi so that the next ATP can be formed. Estimates suggest that for each NADH oxidized, Complexes I and III translocate four protons each and Complex IV translocates two.
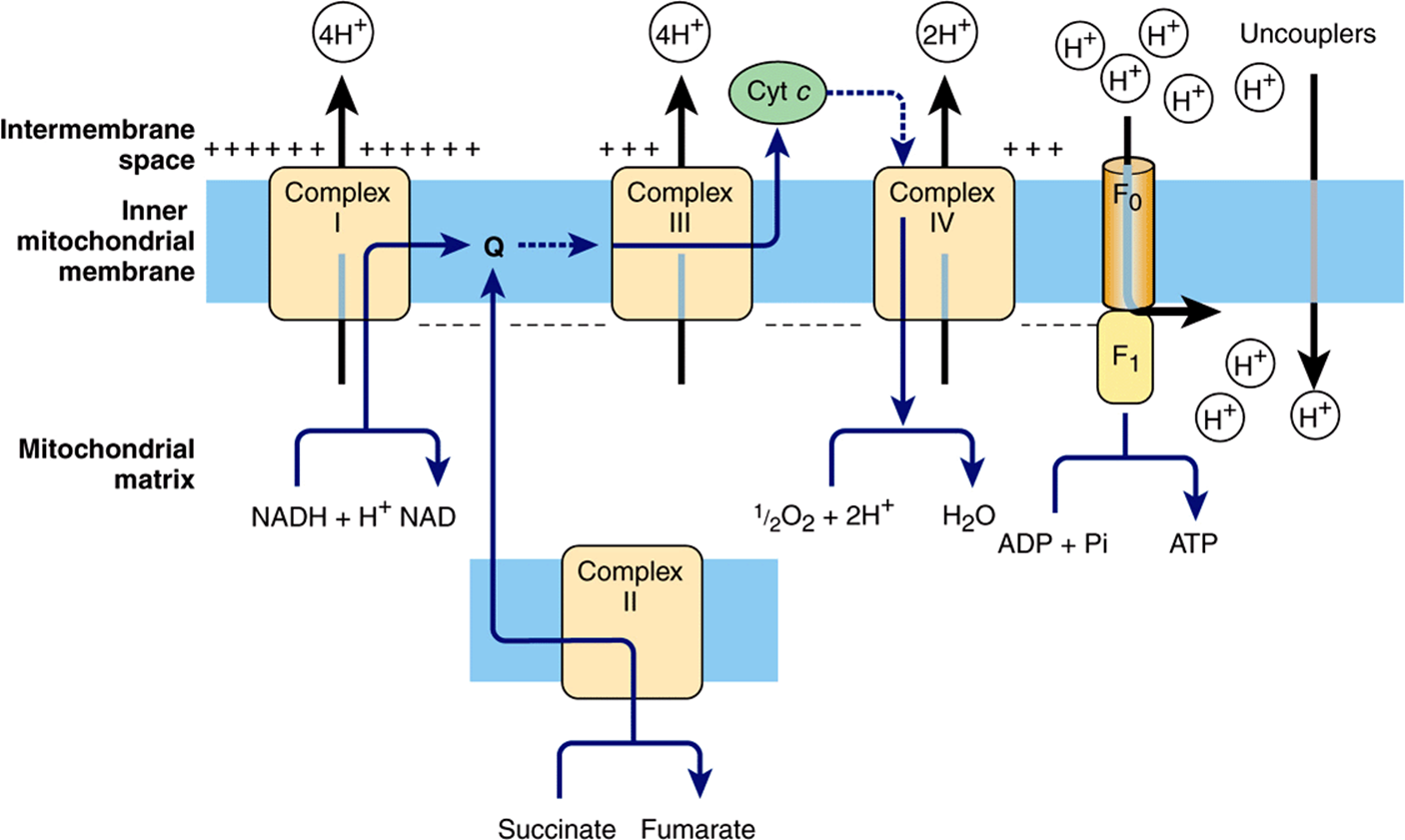
FIGURE 13–7 The chemiosmotic theory of oxidative phosphorylation. Complexes I, III, and IV act as proton pumps creating a proton gradient across the membrane, which is negative on the matrix side. The proton motive force generated drives the synthesis of ATP as the protons flow back into the matrix through the ATP synthase enzyme (see Figure 13–8). Uncouplers increase the permeability of the membrane to ions, collapsing the proton gradient by allowing the H+ to pass across without going through the ATP synthase, and thus uncouple electron flow through the respiratory complexes from ATP synthesis. (cyt, cytochrome; Q, coenzyme Q or ubiquinone.)
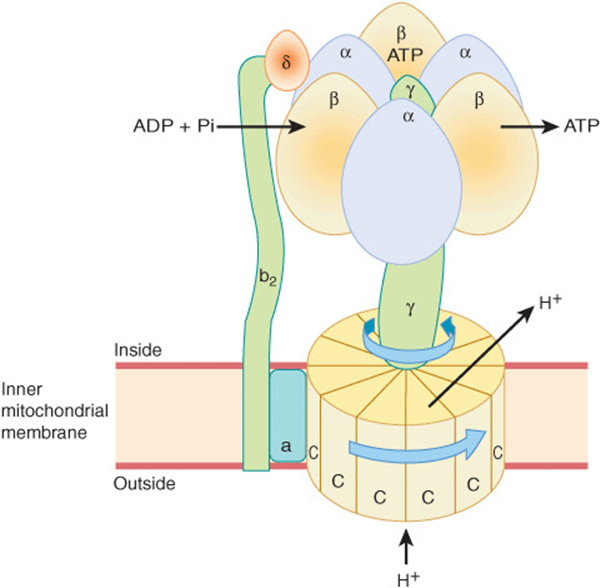
FIGURE 13–8 Mechanism of ATP production by ATP synthase. The enzyme complex consists of an F0 subcomplex which is a disk of “C” protein subunits. Attached is a γ subunit in the form of a “bent axle.” Protons passing through the disk of “C” units cause it and the attached γ subunit to rotate. The γ subunit fits inside the F1 subcomplex of three α and three β subunits, which are fixed to the membrane and do not rotate. ADP and Pi are taken up sequentially by the β subunits to form ATP, which is expelled as the rotating γ subunit squeezes each β subunit in turn and changes its conformation. Thus, three ATP molecules are generated per revolution. For clarity, not all the subunits that have been identified are shown—eg, the “axle” also contains an ε subunit.
THE RESPIRATORY CHAIN PROVIDES MOST OF THE ENERGY CAPTURED DURING CATABOLISM
ADP captures, in the form of high-energy phosphate, a significant proportion of the free energy released by catabolic processes. The resulting ATP has been called the energy “currency” of the cell because it passes on this free energy to drive those processes requiring energy (Figure 11–6).
There is a net direct capture of two high-energy phosphate groups in the glycolytic reactions (Table 18-1). Two more high-energy phosphates per mole of glucose are captured in the citric acid cycle during the conversion of succinyl CoA to succinate. All of these phosphorylations occur at the substrate level. For each mol of substrate oxidized via Complexes I, III, and IV in the respiratory chain (ie, via NADH), 2.5 mol of ATP are formed per 0.5 mol of O2 consumed; ie, the P:O ratio = 2.5 (Figure 13–7). On the other hand, when 1 mol of substrate (eg, succinate or 3-phophoglycerate) is oxidized via Complexes II, III, and IV, only 1.5 mol of ATP are formed; ie, ![]() . These reactions are known as oxidative phosphorylation at the respiratory chain level. Taking these values into account, it can be estimated that nearly 90% of the high-energy phosphates produced from the complete oxidation of 1 mol glucose is obtained via oxidative phosphorylation coupled to the respiratory chain (Table 18-1).
. These reactions are known as oxidative phosphorylation at the respiratory chain level. Taking these values into account, it can be estimated that nearly 90% of the high-energy phosphates produced from the complete oxidation of 1 mol glucose is obtained via oxidative phosphorylation coupled to the respiratory chain (Table 18-1).
Respiratory Control Ensures a Constant Supply of ATP
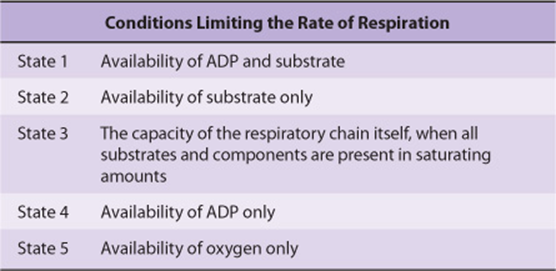
The rate of respiration of mitochondria can be controlled by the availability of ADP. This is because oxidation and phosphorylation are tightly coupled; ie, oxidation cannot proceed via the respiratory chain without concomitant phosphorylation of ADP. Table 13-1 shows the five conditions controlling the rate of respiration in mitochondria. Most cells in the resting state are in state 4, and respiration is controlled by the availability of ADP. When work is performed, ATP is converted to ADP, allowing more respiration to occur, which in turn replenishes the store of ATP. Under certain conditions, the concentration of inorganic phosphate can also affect the rate of functioning of the respiratory chain. As respiration increases (as in exercise), the cell approaches state 3 or 5 when either the capacity of the respiratory chain becomes saturated or the PO2 decreases below the Km for heme a3. There is also the possibility that the ADP/ATP transporter, which facilitates entry of cytosolic ADP into and ATP out of the mitochondrion, becomes rate limiting.
TABLE 13–1 States of Respiratory Control
Thus, the manner in which biologic oxidative processes allow the free energy resulting from the oxidation of foodstuffs to become available and to be captured is stepwise, efficient, and controlled—rather than explosive, inefficient, and uncontrolled, as in many nonbiologic processes. The remaining free energy that is not captured as high-energy phosphate is liberated as heat. This need not to be considered “wasted” since it ensures that the respiratory system as a whole is sufficiently exergonic to be removed from equilibrium, allowing continuous unidirectional flow and constant provision of ATP. It also contributes to maintenance of body temperature.
MANY POISONS INHIBIT THE RESPIRATORY CHAIN
Much information about the respiratory chain has been obtained by the use of inhibitors, and, conversely, this has provided knowledge about the mechanism of action of several poisons (Figure 13–9). They may be classified as inhibitors of the respiratory chain, inhibitors of oxidative phosphorylation, and uncouplers of oxidative phosphorylation.
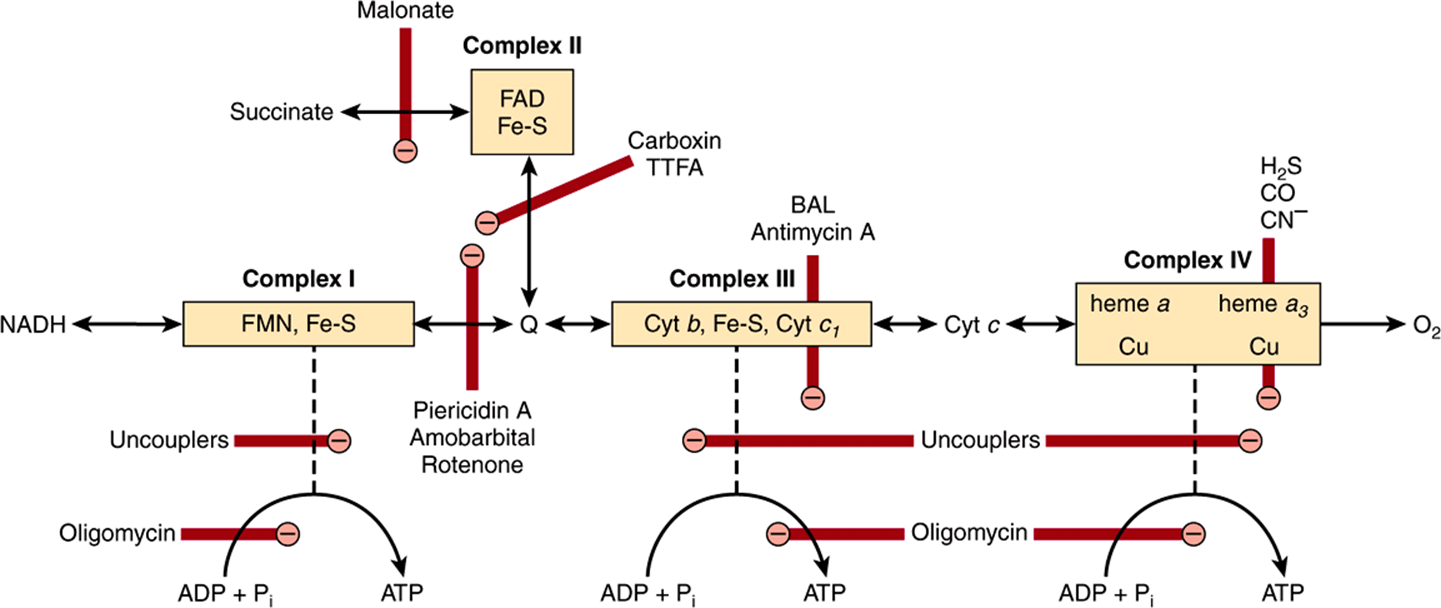
FIGURE 13–9 Sites of inhibition (![]() ) of the respiratory chain by specific drugs, chemicals, and antibiotics.
) of the respiratory chain by specific drugs, chemicals, and antibiotics.
(BAL, dimercaprol; TTFA, an Fe-chelating agent. Other abbreviations as in Figure 13–5.)
Barbiturates such as amobarbital inhibit electron transport via Complex I by blocking the transfer from Fe-S to Q. At sufficient dosage, they are fatal in vivo. Antimycin A and dimercaprol inhibit the respiratory chain at Complex III. The classic poisons H2S, carbon monoxide, and cyanide inhibit Complex IV and can therefore totally arrest respiration. Malonate is a competitive inhibitor of Complex II.
Atractyloside inhibits oxidative phosphorylation by inhibiting the transporter of ADP into and ATP out of the mitochondrion (Figure 13–10). The antibiotic oligomycin completely blocks oxidation and phosphorylation by blocking the flow of protons through ATP synthase (Figure 13–9).
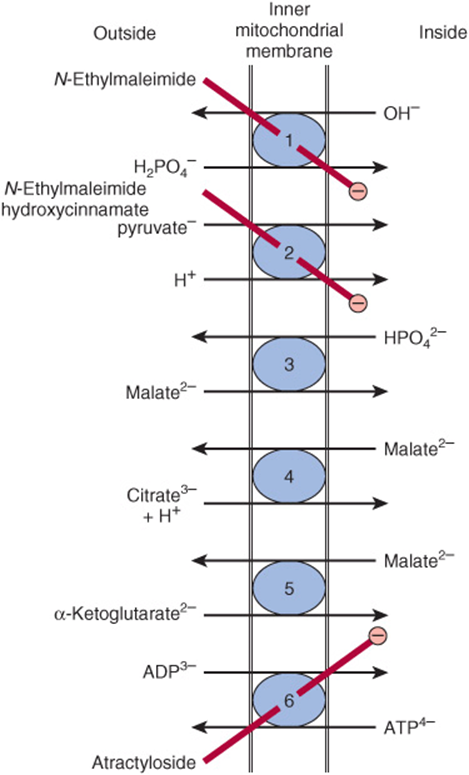
FIGURE 13–10 Transporter systems in the inner mitochondrial membrane. ![]() Phosphate transporter,
Phosphate transporter, ![]() pyruvate symport,
pyruvate symport, ![]() dicarboxylate transporter,
dicarboxylate transporter, ![]() tricarboxylate transporter,
tricarboxylate transporter, ![]() α-ketoglutarate transporter,
α-ketoglutarate transporter, ![]() adenine nucleotide transporter. N-Ethylmaleimide, hydroxycinnamate, and atractyloside inhibit (
adenine nucleotide transporter. N-Ethylmaleimide, hydroxycinnamate, and atractyloside inhibit (![]() ) the indicated systems. Also present (but not shown) are transporter systems for glutamate/aspartate (Figure 13–13), glutamine, ornithine, neutral amino acids, and carnitine (Figure 22–1).
) the indicated systems. Also present (but not shown) are transporter systems for glutamate/aspartate (Figure 13–13), glutamine, ornithine, neutral amino acids, and carnitine (Figure 22–1).
Uncouplers dissociate oxidation in the respiratory chain from phosphorylation (Figure 13–7). These compounds are toxic in vivo, causing respiration to become uncontrolled, since the rate is no longer limited by the concentration of ADP or Pi. The uncoupler that has been used most frequently is 2,4-dinitrophenol, but other compounds act in a similar manner. Thermogenin (or the uncoupling protein) is a physiological uncoupler found in brown adipose tissue that functions to generate body heat, particularly for the newborn and during hibernation in animals (Chapter 25).
THE CHEMIOSMOTIC THEORY CAN ACCOUNT FOR RESPIRATORY CONTROL AND THE ACTION OF UNCOUPLERS
The electrochemical potential difference across the membrane, once established as a result of proton translocation, inhibits further transport of reducing equivalents through the respiratory chain unless discharged by back-translocation of protons across the membrane through the ATP synthase. This in turn depends on availability of ADP and Pi.
Uncouplers (eg, dinitrophenol) are amphipathic (Chapter 15) and increase the permeability of the lipoid inner mitochondrial membrane to protons, thus reducing the electrochemical potential and short-circuiting the ATP synthase (Figure 13–7). In this way, oxidation can proceed without phosphorylation.
THE RELATIVE IMPERMEABILITY OF THE INNER MITOCHONDRIAL MEMBRANE NECESSITATES EXCHANGE TRANSPORTERS
Exchange diffusion systems involving transporter proteins that span the membrane are present in the membrane for exchange of anions against OH– ions and cations against H+ ions. Such systems are necessary for uptake and output of ionized metabolites while preserving electrical and osmotic equilibrium. The inner mitochondrial membrane is freely permeable to uncharged small molecules, such as oxygen, water, CO2, NH3, and to monocarboxylic acids, such as 3-hydroxybutyric, acetoacetic, and acetic. Long-chain fatty acids are transported into mitochondria via the carnitine system (Figure 22–1), and there is also a special carrier for pyruvate involving a symport that utilizes the H+ gradient from outside to inside the mitochondrion (Figure 13–10). However, dicarboxylate and tricarboxylate anions and amino acids require specific transporter or carrier systems to facilitate their passage across the membrane. Monocarboxylic acids penetrate more readily in their undissociated, more lipid-soluble form.
The transport of di- and tricarboxylate anions is closely linked to that of inorganic phosphate, which penetrates readily as the ![]() ion in exchange for OH–. The net uptake of malate by the dicarboxylate transporter requires inorganic phosphate for exchange in the opposite direction. The net uptake of citrate, isocitrate, or cis-aconitate by the tricarboxylate transporter requires malate in exchange. α-Ketoglutarate transport also requires an exchange with malate. The adenine nucleotide transporter allows the exchange of ATP and ADP but not AMP. It is vital in allowing ATP exit from mitochondria to the sites of extramitochondrial utilization and in allowing the return of ADP for ATP production within the mitochondrion (Figure 13–11). Since in this translocation four negative charges are removed from the matrix for every three taken in, the electrochemical gradient across the membrane (the proton motive force) favors the export of ATP. Na+ can be exchanged for H+, driven by the proton gradient. It is believed that active uptake of Ca2+ by mitochondria occurs with a net charge transfer of 1 (Ca2+ uniport), possibly through a Ca2+/H+antiport. Calcium release from mitochondria is facilitated by exchange with Na+.
ion in exchange for OH–. The net uptake of malate by the dicarboxylate transporter requires inorganic phosphate for exchange in the opposite direction. The net uptake of citrate, isocitrate, or cis-aconitate by the tricarboxylate transporter requires malate in exchange. α-Ketoglutarate transport also requires an exchange with malate. The adenine nucleotide transporter allows the exchange of ATP and ADP but not AMP. It is vital in allowing ATP exit from mitochondria to the sites of extramitochondrial utilization and in allowing the return of ADP for ATP production within the mitochondrion (Figure 13–11). Since in this translocation four negative charges are removed from the matrix for every three taken in, the electrochemical gradient across the membrane (the proton motive force) favors the export of ATP. Na+ can be exchanged for H+, driven by the proton gradient. It is believed that active uptake of Ca2+ by mitochondria occurs with a net charge transfer of 1 (Ca2+ uniport), possibly through a Ca2+/H+antiport. Calcium release from mitochondria is facilitated by exchange with Na+.
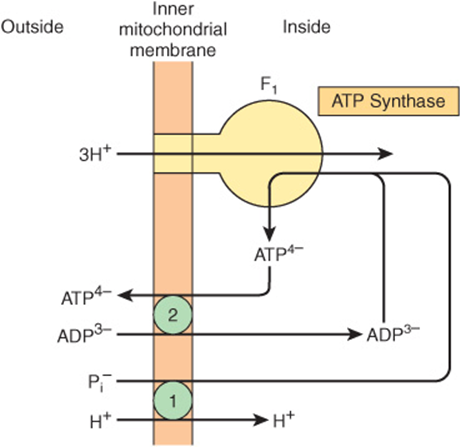
FIGURE 13–11 Combination of phosphate transporter ![]() with the adenine nucleotide transporter
with the adenine nucleotide transporter ![]() in ATP synthesis. The H+/Pi symport shown is equivalent to the Pi/OH– antiport shown in Figure 13–10.
in ATP synthesis. The H+/Pi symport shown is equivalent to the Pi/OH– antiport shown in Figure 13–10.
Ionophores Permit Specific Cations to Penetrate Membranes
Ionophores are lipophilic molecules that complex specific cations and facilitate their transport through biologic membranes, eg, valinomycin (K+). The classic uncouplers such as dinitrophenol are, in fact, proton ionophores.
A Proton-Translocating Transhydrogenase Is a Source of Intramitochondrial NADPH
Energy-linked transhydrogenase, a protein in the inner mitochondrial membrane, couples the passage of protons down the electrochemical gradient from outside to inside the mitochondrion with the transfer of H from intramitochondrial NADH to NADPH for intramitochondrial enzymes such as glutamate dehydrogenase and hydroxylases involved in steroid synthesis.
Oxidation of Extramitochondrial NADH Is Mediated by Substrate Shuttles
NADH cannot penetrate the mitochondrial membrane, but it is produced continuously in the cytosol by 3-phosphoglyceraldehyde dehydrogenase, an enzyme in the glycolysis sequence (Figure 18–2). However, under aerobic conditions, extramitochondrial NADH does not accumulate and is presumed to be oxidized by the respiratory chain in mitochondria. The transfer of reducing equivalents through the mitochon-drial membrane requires substrate pairs, linked by suitable dehydrogenases on each side of the mitochondrial membrane. The mechanism of transfer using the glycerophosphate shuttle is shown in Figure 13–12. Since the mitochondrial enzyme is linked to the respiratory chain via a flavoprotein rather than NAD, only 1.5 mol rather than 2.5 mol of ATP are formed per atom of oxygen consumed. Although this shuttle is present in some tissues (eg, brain, white muscle), in others (eg, heart muscle) it is deficient. It is therefore believed that the malate shuttle system (Figure 13–13, is of more universal utility. The complexity of this system is due to the impermeability of the mitochondrial membrane to oxaloacetate, which must react with glutamate to form aspartate and α-ketoglutarate by transamination before transport through the mitochondrial membrane and reconstitution to oxaloacetate in the cytosol.
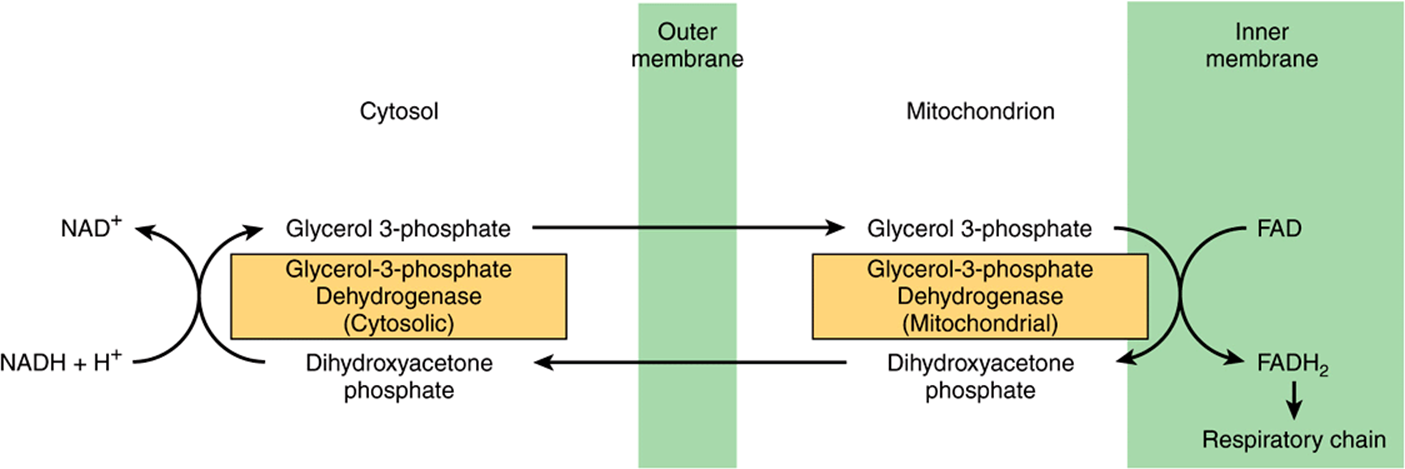
FIGURE 13–12 Glycerophosphate shuttle for transfer of reducing equivalents from the cytosol into the mitochondrion.
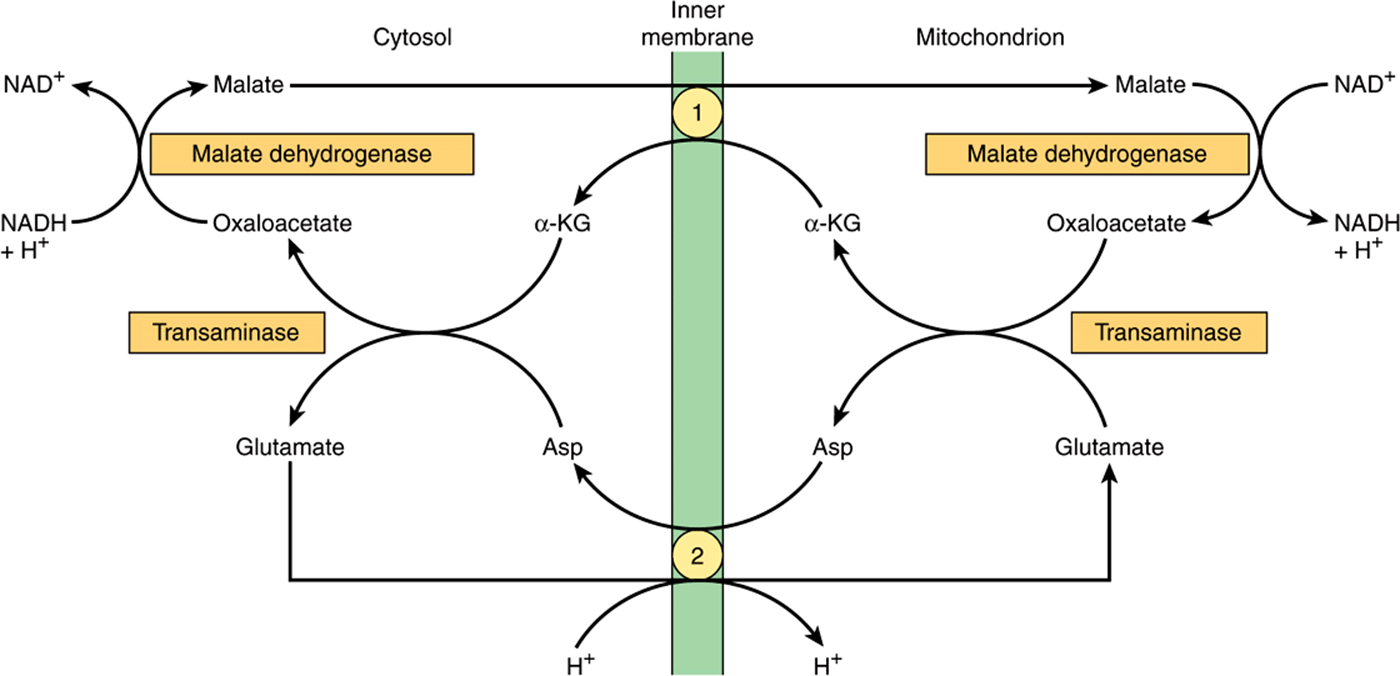
FIGURE 13–13 Malate shuttle for transfer of reducing equivalents from the cytosol into the mitochondrion. ![]() α-Ketoglutarate transporter and
α-Ketoglutarate transporter and ![]() glutamate/aspartate transporter (note the proton symport with glutamate).
glutamate/aspartate transporter (note the proton symport with glutamate).
Ion Transport in Mitochondria Is Energy Linked
Mitochondria maintain or accumulate cations such as K+, Na+, Ca2+, and Mg2+, and Pi. It is assumed that a primary proton pump drives cation exchange.
The Creatine Phosphate Shuttle Facilitates Transport of High-Energy Phosphate from Mitochondria
This shuttle (Figure 13–14) augments the functions of creatine phosphate as an energy buffer by acting as a dynamic system for transfer of high-energy phosphate from mitochondria in active tissues such as heart and skeletal muscle. An isoenzyme of creatine kinase (CKm) is found in the mitochondrial intermembrane space, catalyzing the transfer of high-energy phosphate to creatine from ATP emerging from the adenine nucleotide transporter. In turn, the creatine phosphate is transported into the cytosol via protein pores in the outer mitochondrial membrane, becoming available for generation of extramitochondrial ATP.
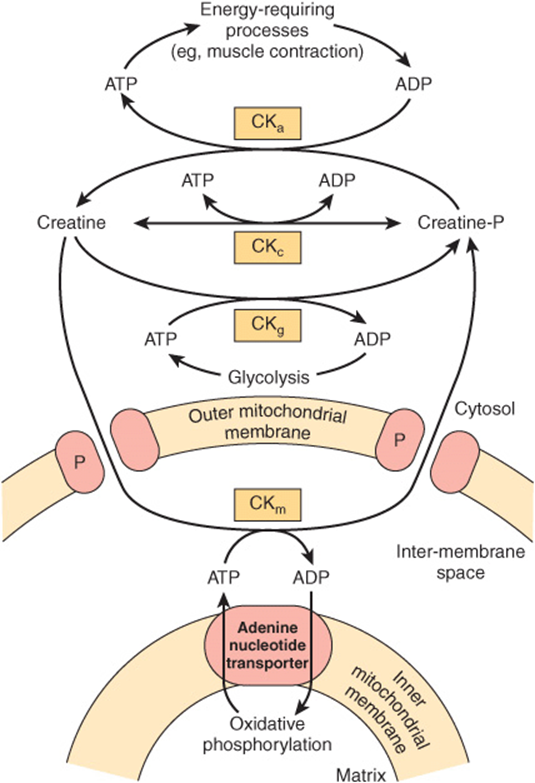
FIGURE 13–14 The creatine phosphate shuttle of heart and skeletal muscle. The shuttle allows rapid transport of high-energy phosphate from the mitochondrial matrix into the cytosol. (CKa, creatine kinase concerned with large requirements for ATP, eg, muscular contraction; CKc, creatine kinase for maintaining equilibrium between creatine and creatine phosphate and ATP/ADP; CKg, creatine kinase coupling glycolysis to creatine phosphate synthesis; CKm, mitochondrial creatine kinase mediating creatine phosphate production from ATP formed in oxidative phosphorylation; P, pore protein in outer mitochondrial membrane.)
CLINICAL ASPECTS
The condition known as fatal infantile mitochondrial myopathy and renal dysfunction involves severe diminution or absence of most oxidoreductases of the respiratory chain. MELAS (mitochondrial encephalopathy, lactic acidosis, and stroke) is an inherited condition due to NADH-Q oxidoreductase (Complex I) or cytochrome oxidase (Complex IV) deficiency. It is caused by a mutation in mitochondrial DNA and may be involved in Alzheimer’s disease and diabetes mellitus. A number of drugs and poisons act by inhibition of oxidative phosphorylation.
SUMMARY
![]() Virtually all energy released from the oxidation of carbohydrate, fat, and protein is made available in mitochondria as reducing equivalents (—H or e–). These are funneled into the respiratory chain, where they are passed down a redox gradient of carriers to their final reaction with oxygen to form water.
Virtually all energy released from the oxidation of carbohydrate, fat, and protein is made available in mitochondria as reducing equivalents (—H or e–). These are funneled into the respiratory chain, where they are passed down a redox gradient of carriers to their final reaction with oxygen to form water.
![]() The redox carriers are grouped into four respiratory chain complexes in the inner mitochondrial membrane. Three of the four complexes are able to use the energy released in the redox gradient to pump protons to the outside of the membrane, creating an electrochemical potential between the matrix and the inner membrane space.
The redox carriers are grouped into four respiratory chain complexes in the inner mitochondrial membrane. Three of the four complexes are able to use the energy released in the redox gradient to pump protons to the outside of the membrane, creating an electrochemical potential between the matrix and the inner membrane space.
![]() ATP synthase spans the membrane and acts like a rotary motor using the potential energy of the proton gradient or proton motive force to synthesize ATP from ADP and Pi. In this way, oxidation is closely coupled to phosphorylation to meet the energy needs of the cell.
ATP synthase spans the membrane and acts like a rotary motor using the potential energy of the proton gradient or proton motive force to synthesize ATP from ADP and Pi. In this way, oxidation is closely coupled to phosphorylation to meet the energy needs of the cell.
![]() Since the inner mitochondrial membrane is impermeable to protons and other ions, special exchange transporters span the membrane to allow ions such as OH–, ATP4-, ADP3-, and metabolites to pass through without discharging the electrochemical gradient across the membrane.
Since the inner mitochondrial membrane is impermeable to protons and other ions, special exchange transporters span the membrane to allow ions such as OH–, ATP4-, ADP3-, and metabolites to pass through without discharging the electrochemical gradient across the membrane.
![]() Many well-known poisons such as cyanide arrest respiration by inhibition of the respiratory chain.
Many well-known poisons such as cyanide arrest respiration by inhibition of the respiratory chain.
REFERENCES
Hinkle PC: P/O ratios of mitochondrial oxidative phosphorylation. Biochem Biophys Acta 2005;1706:1.
Kocherginsky N: Acidic lipids, H(+)-ATPases, and mechanism of oxidative phosphorylation. Physico-chemical ideas 30 years after P. Mitchell’s Nobel Prize award. Prog Biophys Mol Biol 2009;99:20.
Mitchell P: Keilin’s respiratory chain concept and its chemiosmotic consequences. Science 1979;206:1148.
Nakamoto RK, Baylis Scanlon JA, Al-Shawi MK: The rotary mechanism of the ATP synthase. Arch Biochem Biophys 2008;476:43.
Smeitink J, van den Heuvel L, DiMauro S: The genetics and pathology of oxidative phosphorylation. Nat Rev Genet 2001;2:342.
Tyler DD: The Mitochondrion in Health and Disease. VCH Publishers, 1992.
Wallace DC: Mitochondrial DNA in aging and disease. Sci Am 1997;277:22.
Yoshida M, Muneyuki E, Hisabori T: ATP synthase—a marvelous rotary engine of the cell. Nat Rev Mol Cell Biol 2001;2:669.