Harper’s Illustrated Biochemistry, 29th Edition (2012)
SECTION VI. Special Topics
Chapter 44. Micronutrients: Vitamins & Minerals
David A. Bender, PhD
OBJECTIVES
After studying this chapter, you should be able to:
![]() Describe how reference intakes for vitamins and minerals are determined and explain why reference intakes published by different national and international authorities differ.
Describe how reference intakes for vitamins and minerals are determined and explain why reference intakes published by different national and international authorities differ.
![]() Define a vitamin and describe the metabolism, principal functions, deficiency diseases associated with inadequate intake, and the toxicity of excessive intakes of the vitamins.
Define a vitamin and describe the metabolism, principal functions, deficiency diseases associated with inadequate intake, and the toxicity of excessive intakes of the vitamins.
![]() Explain why mineral salts are required in the diet.
Explain why mineral salts are required in the diet.
BIOMEDICAL IMPORTANCE
Vitamins are a group of organic nutrients, required in small quantities for a variety of biochemical functions that, generally, cannot be synthesized by the body and must therefore be supplied in the diet.
The lipid-soluble vitamins are hydrophobic compounds that can be absorbed efficiently only when there is normal fat absorption. Like other lipids, they are transported in the blood in lipoproteins or attached to specific binding proteins. They have diverse functions—eg, vitamin A, vision and cell differentiation; vitamin D, calcium and phosphate metabolism, and cell differentiation; vitamin E, antioxidant; and vitamin K, blood clotting. As well as dietary inadequacy, conditions affecting the digestion and absorption of the lipid-soluble vitamins, such as a very low fat diet, steatorrhea and disorders of the biliary system, can all lead to deficiency syndromes, including night blindness and xerophthalmia (vitamin A); rickets in young children and osteomalacia in adults (vitamin D); neurologic disorders and hemolytic anemia of the newborn (vitamin E); and hemorrhagic disease of the newborn (vitamin K). Toxicity can result from excessive intake of vitamins A and D. Vitamin A and the carotenes (many of which are precursors of vitamin A), and vitamin E are antioxidants (Chapter 45) and have possible roles in prevention of atherosclerosis and cancer.
The water-soluble vitamins are vitamins B and C; they function mainly as enzyme cofactors. Folic acid acts as a carrier of one-carbon units. Deficiency of a single vitamin of the B complex is rare since poor diets are most often associated with multiple deficiency states. Nevertheless, specific syndromes are characteristic of deficiencies of individual vitamins, eg, beriberi (thiamin); cheilosis, glossitis, seborrhea (riboflavin); pellagra (niacin); megaloblastic anemia, methylmalonic aciduria, and pernicious anemia (vitamin B); megaloblastic anemia (folic acid); and scurvy (vitamin C).
Inorganic mineral elements that have a function in the body must be provided in the diet. When the intake is insufficient, deficiency signs may arise, eg, anemia (iron), and cretinism and goiter (iodine). Excessive intakes may be toxic.
The Determination of Micronutrient Requirements Depends on the Criteria of Adequacy Chosen
For any nutrient, there is a range of intakes between that which is clearly inadequate, leading to clinical deficiency disease, and that which is so much in excess of the body’s metabolic capacity that there may be signs of toxicity.Between these two extremes is a level of intake that is adequate for normal health and the maintenance of metabolic integrity. Individuals do not all have the same requirement for nutrients, even when calculated on the basis of body size or energy expenditure. There is a range of individual requirements of up to 25% around the mean. Therefore, in order to assess the adequacy of diets, it is necessary to set a reference level of intake high enough to ensure that no one either suffers from deficiency or is at risk of toxicity. If it is assumed that individual requirements are distributed in a statistically normal fashion around the observed mean requirement, then a range of ±2 × the standard deviation (SD) around the mean includes the requirements of 95% of the population. Reference or recommended intakes are therefore set at the average requirement plus 2 × SD, and so meet or exceed the requirements of 97.5% of the population.
Reference and recommended intakes of vitamins and minerals published by different national and international authorities (Tables 44-1 to 44-4) differ because of different interpretations of the available data, and the availability of new experimental data in more recent publications.
TABLE 44–1 Reference Nutrient Intakes of Vitamins and Minerals, UK 1991
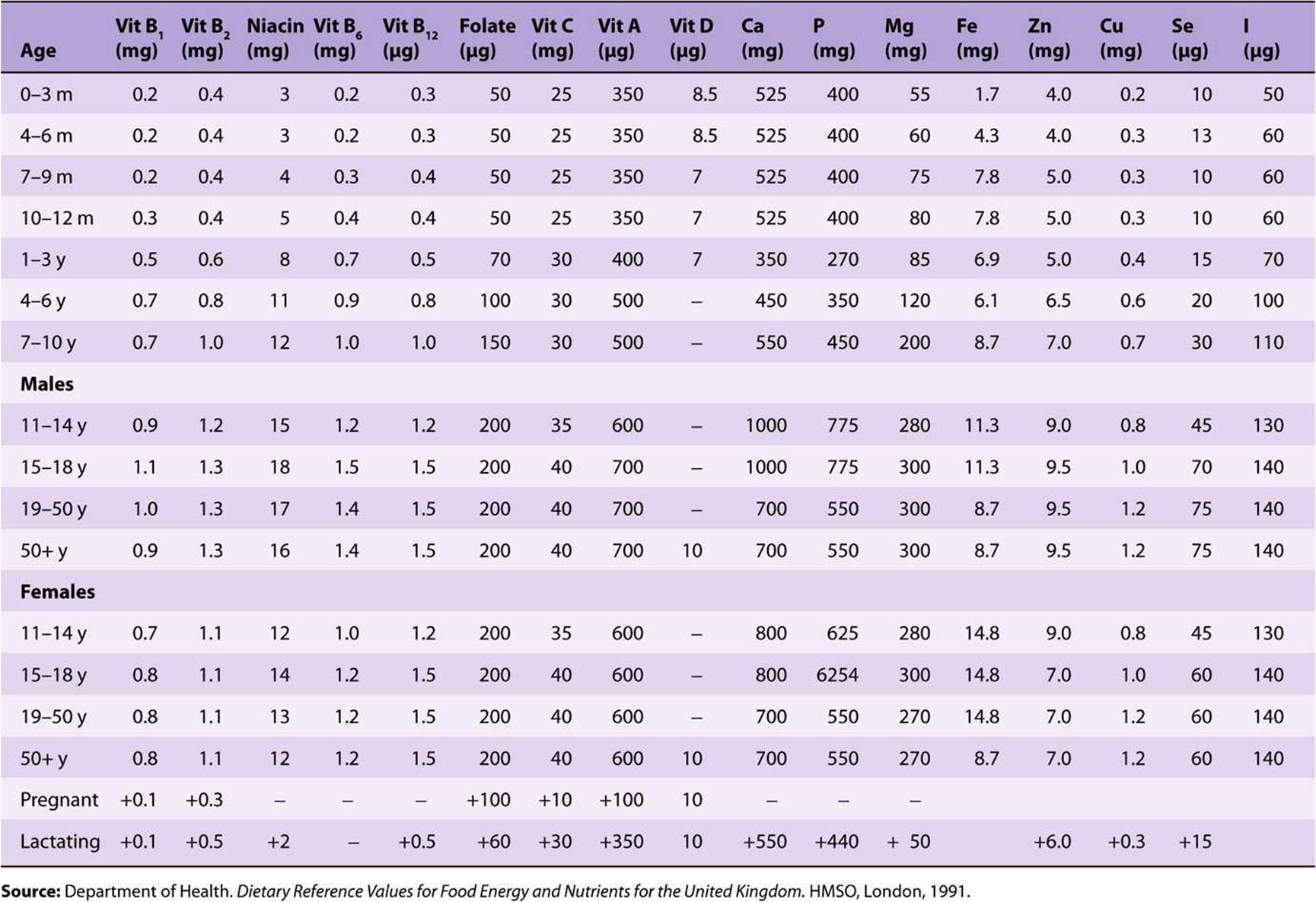
TABLE 44–2 Population Reference Intakes of Vitamins and Minerals, European Union, 1993
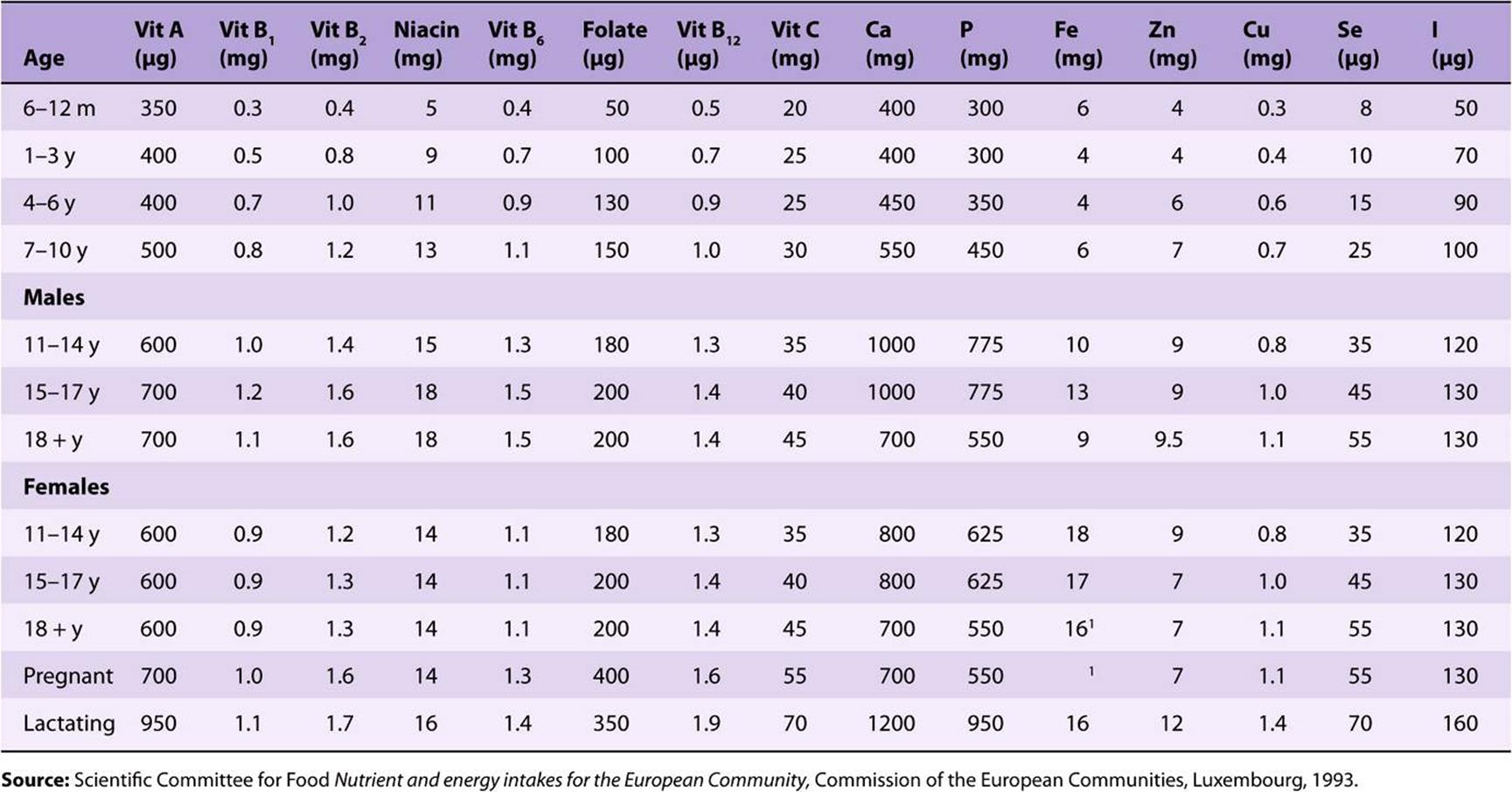
TABLE 44–3 Recommended Dietary Allowances and Acceptable Intakes for Vitamins and Minerals, USA and Canada, 1997-2001
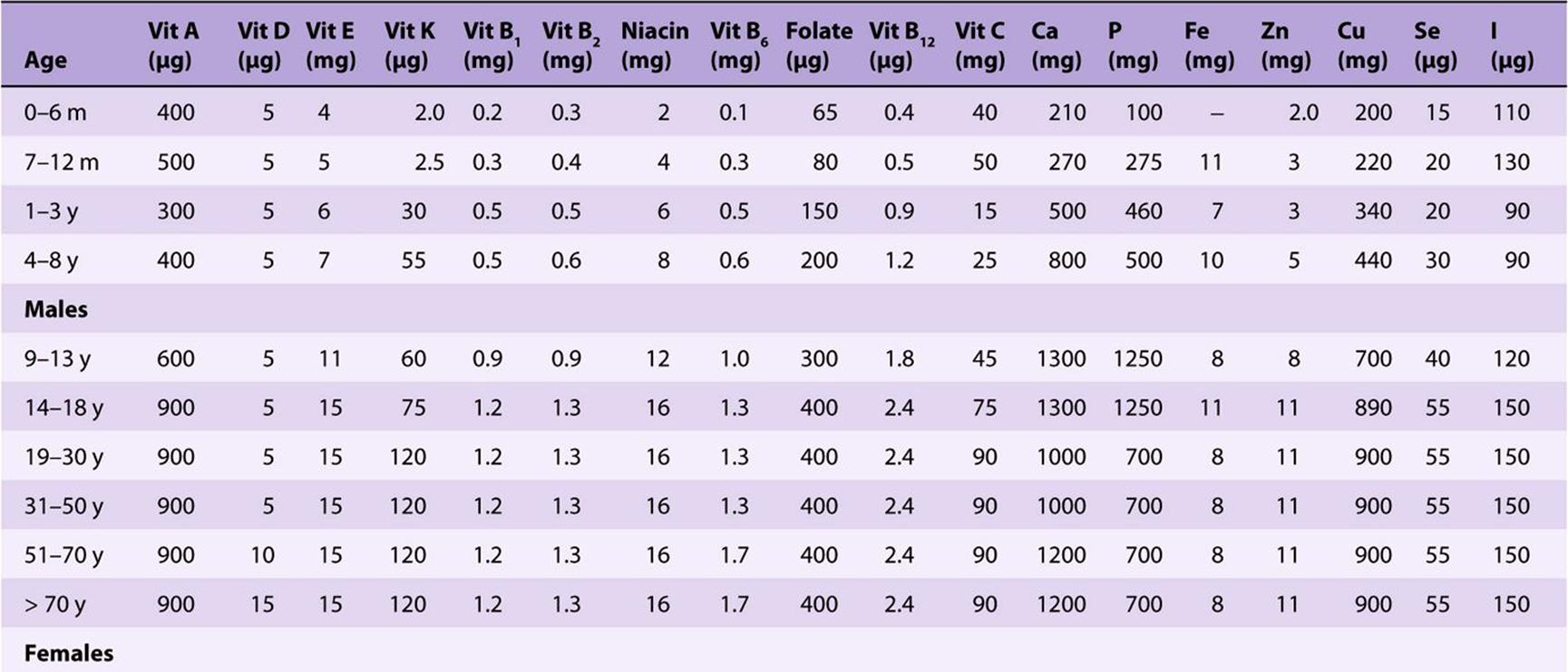
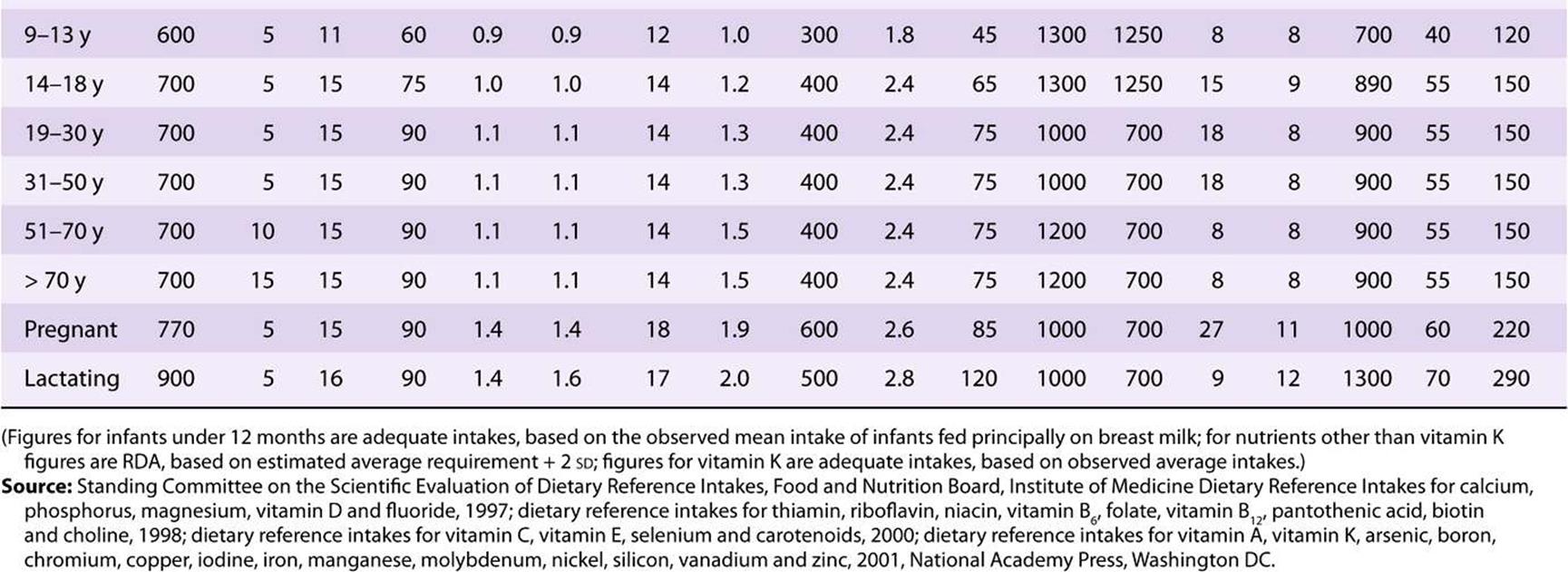
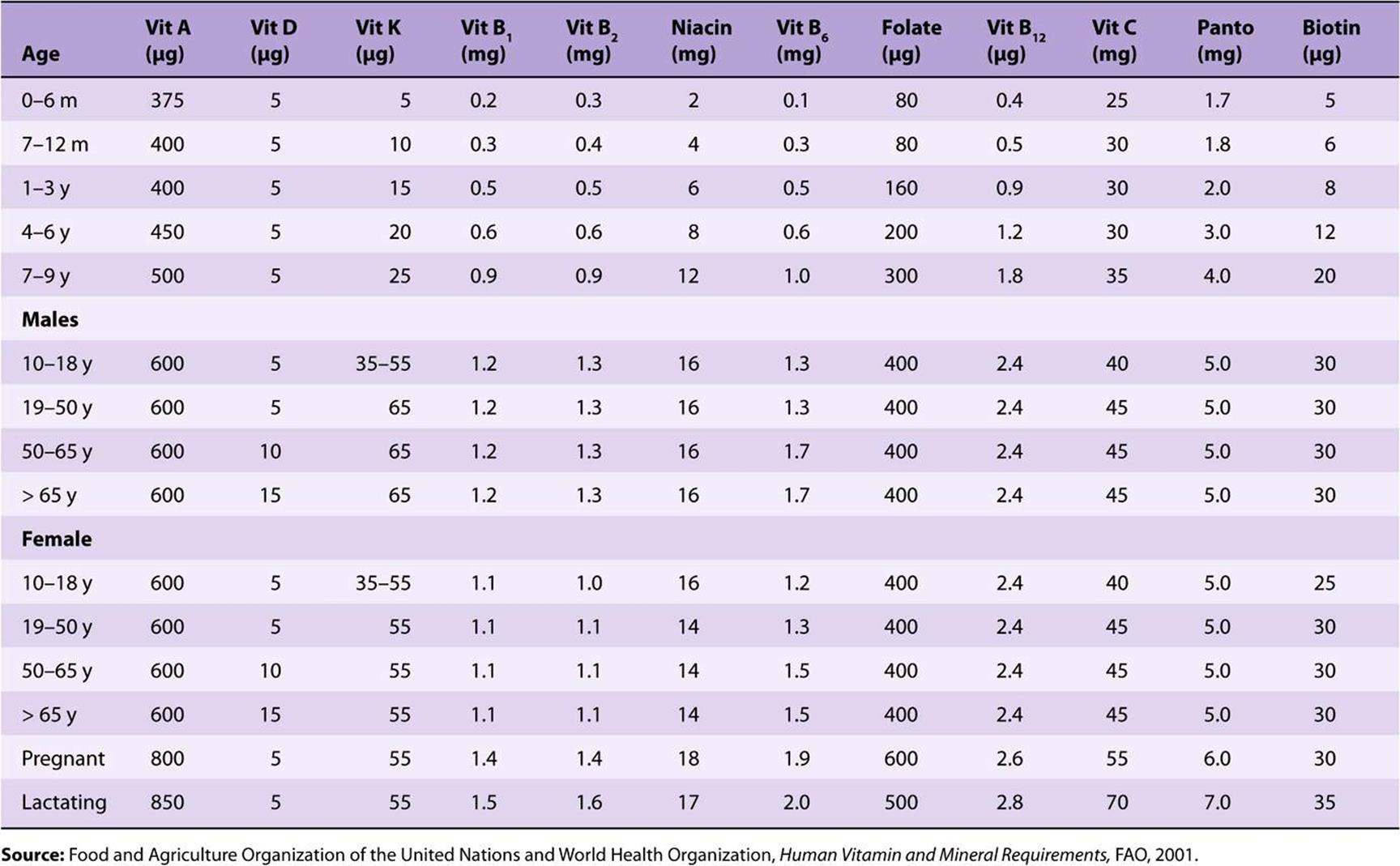
TABLE 44–4 Recommended Nutrient Intakes for Vitamins, FAO 2001
THE VITAMINS ARE A DISPARATE GROUP OF COMPOUNDS WITH A VARIETY OF METABOLIC FUNCTIONS
A vitamin is defined as an organic compound that is required in the diet in small amounts for the maintenance of normal metabolic integrity. Deficiency causes a specific disease, which is cured or prevented only by restoring the vitamin to the diet (Table 44-5). However, vitamin D, which is formed in the skin from 7-dehydrocholesterol on exposure to sunlight, and niacin, which can be formed from the essential amino acid tryptophan, do not strictly comply with this definition.
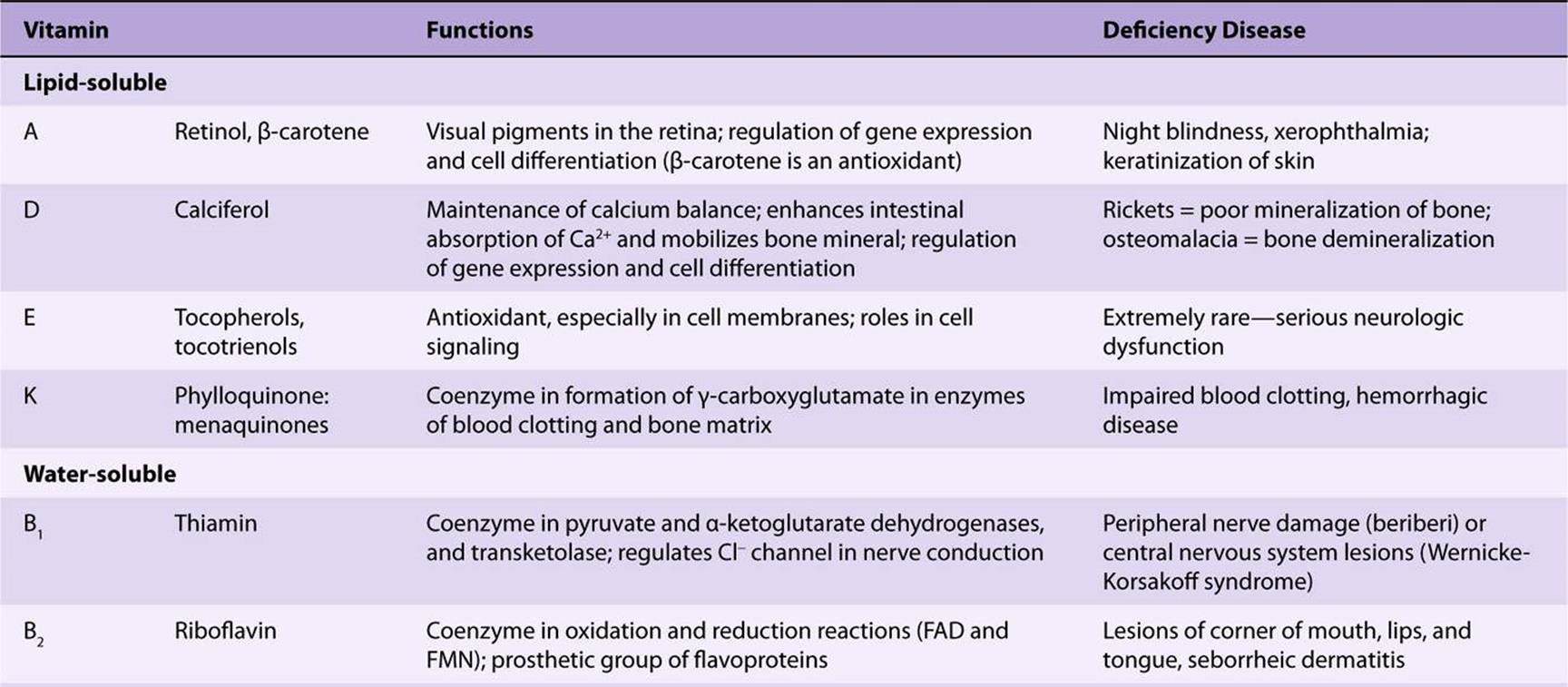
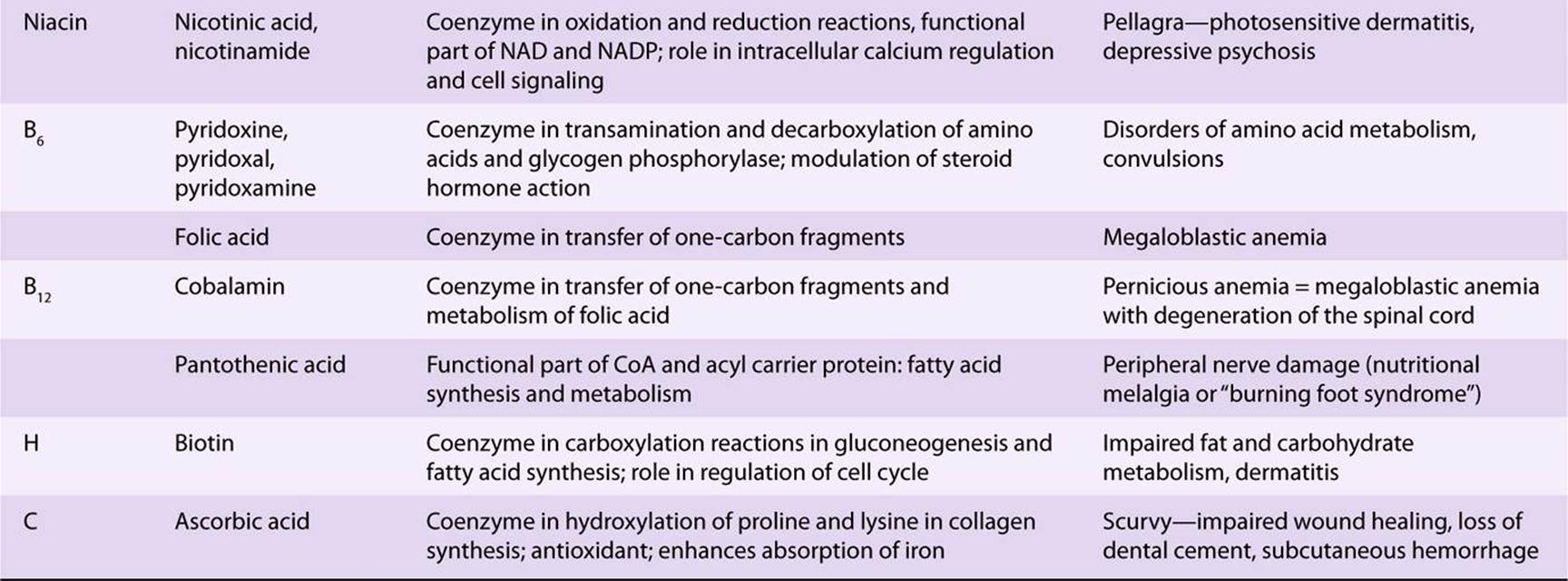
TABLE 44–5 The Vitamins
LIPID-SOLUBLE VITAMINS
TWO GROUPS OF COMPOUNDS HAVE VITAMIN A ACTIVITY
Retinoids comprise retinol, retinaldehyde, and retinoic acid (preformed vitamin A, found only in foods of animal origin); carotenoids, found in plants, are composed of carotenes and related compounds; many are precursors of vitamin A, as they can be cleaved to yield retinaldehyde, then retinol and retinoic acid (Figure 44–1). The α-, β-, and γ-carotenes and cryptoxanthin are quantitatively the most important provitamin A carotenoids. β-Carotene and other provitamin A carotenoids are cleaved in the intestinal mucosa by carotene dioxygenase, yielding retinaldehyde, which is reduced to retinol, esterified and secreted in chylomicrons together with esters formed from dietary retinol. The intestinal activity of carotene dioxygenase is low, so that a relatively large proportion of ingested β-carotene may appear in the circulation unchanged. While the principal site of carotene dioxygenase attack is the central bond of β-carotene, asymmetric cleavage may also occur, leading to the formation of 8′-, 10′-, and 12′-apo-carotenals, which are oxidized to retinoic acid, but cannot be used as sources of retinol or retinaldehyde.
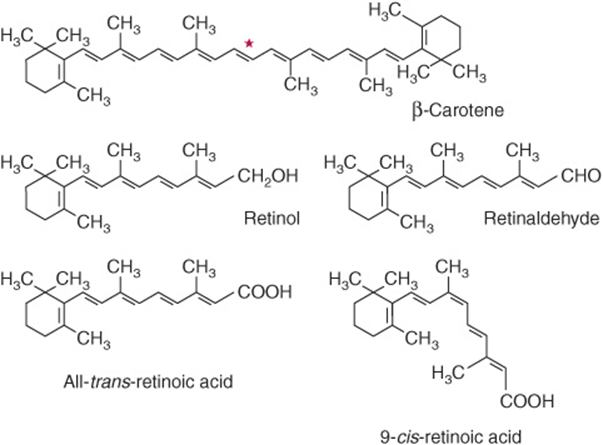
FIGURE 44–1 β-Carotene and the major vitamin A vitamers. Asterisk shows the site of cleavage of β-carotene by carotene dioxygenase, to yield retinaldehyde.
Although it would appear that one molecule of β-carotene should yield two of retinol, this is not so in practice; 6 μg of β-carotene is equivalent to 1 μg of preformed retinol. The total amount of vitamin A in foods is therefore expressed as micrograms of retinol equivalents = μg preformed vitamin A + 1/6 × μg β-carotene + 1/12 × μg other provitamin A carotenoids. Before pure vitamin A was available for chemical analysis, the vitamin A content of foods was determined by biological assay and the results expressed as international units (iu). 1 iu = 0.3 μg retinol; 1 μg retinol = 3.33 iu. Although obsolete, iu is sometimes still used in food labeling. In 2001 The USA/Canadian Dietary Reference Values report introduced the term retinol activity equivalent to take account of the incomplete absorption and metabolism of carotenoids; 1 ![]() μg all-trans-retinol, 12 μg β-carotene, 24 μg α-carotene or β-cryptoxanthin. On this basis, 1 iu of vitamin A activity is equal to 3.6 μg β-carotene or 7.2 μg of other provitamin A carotenoids.
μg all-trans-retinol, 12 μg β-carotene, 24 μg α-carotene or β-cryptoxanthin. On this basis, 1 iu of vitamin A activity is equal to 3.6 μg β-carotene or 7.2 μg of other provitamin A carotenoids.
Vitamin A Has a Function in Vision
In the retina, retinaldehyde functions as the prosthetic group of the light-sensitive opsin proteins, forming rhodopsin (in rods) and iodopsin (in cones). Any one cone cell contains only one type of opsin and is sensitive to only one color. In the pigment epithelium of the retina, all-trans-retinol is isomerized to 11-cis-retinol and oxidized to 11-cis-retinaldehyde. This reacts with a lysine residue in opsin, forming the holoprotein rhodopsin. As shown in Figure 44–2, the absorption of light by rhodopsin causes isomerization of the retinaldehyde from 11-cis to all-trans, and a conformational change in opsin. This results in the release of retinaldehyde from the protein, and the initiation of a nerve impulse. The formation of the initial excited form of rhodopsin, bathorhodopsin, occurs within picoseconds of illumination. There is then a series of conformational changes leading to the formation of metarhodopsin II, which initiates a guanine nucleotide amplification cascade and then a nerve impulse. The final step is hydrolysis to release all-trans-retinaldehyde and opsin. The key to initiation of the visual cycle is the availability of 11-cis-retinaldehyde, and hence vitamin A. In deficiency, both the time taken to adapt to darkness and the ability to see in poor light are impaired.
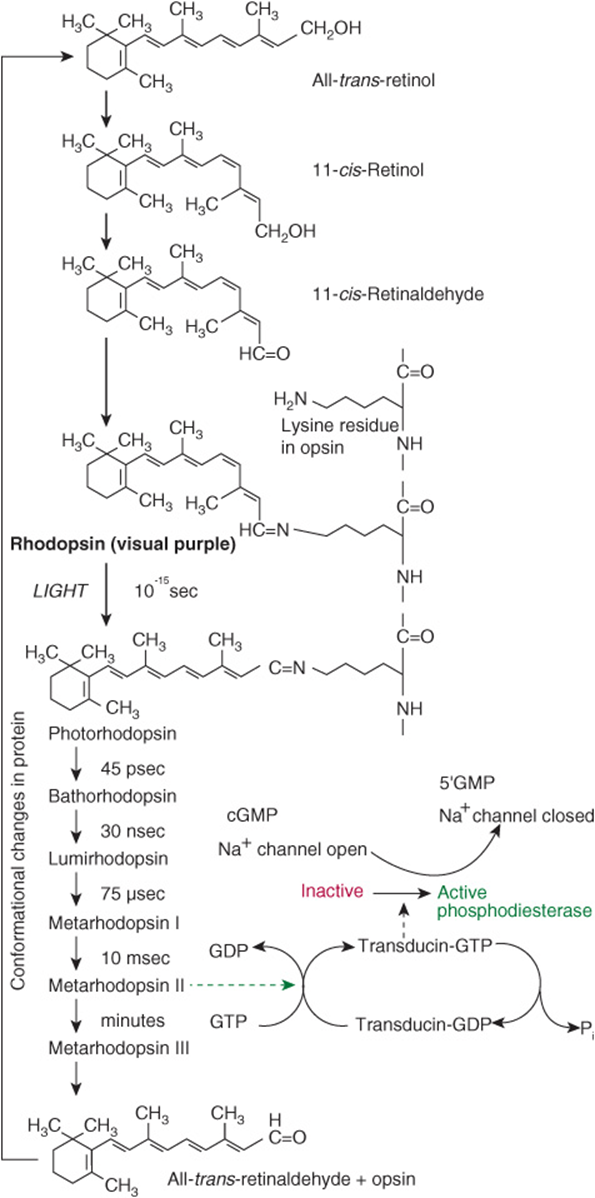
FIGURE 44–2 The role of retinaldehyde in the visual cycle.
Retinoic Acid Has a Role in the Regulation of Gene Expression and Tissue Differentiation
A major role of vitamin A is in the control of cell differentiation and turnover. All-trans-retinoic acid and 9-cis-retinoic acid (Figure 44–1) regulate growth, development, and tissue differentiation; they have different actions in different tissues. Like the thyroid and steroid hormones and vitamin D, retinoic acid binds to nuclear receptors that bind to response elements of DNA and regulate the transcription of specific genes. There are two families of nuclear retinoid receptors: the retinoic acid receptors (RAR) bind all-trans-retinoic acid or 9-cis-retinoic acid, and the retinoid X receptors (RXR) bind 9-cis-retinoic acid. Retinoid X receptors also form dimers with vitamin D, thyroid, and other a nuclear acting hormone receptors. Deficiency of vitamin A impairs vitamin D function because of lack of 9-cis-retinoic acid to form receptor dimers, while excessive vitamin A also impairs vitamin D function, because of formation of RXR homodimers, meaning that there are not enough RXR available to form heterodimers with the vitamin D receptor.
Vitamin A Deficiency Is a Major Public Health Problem Worldwide
Vitamin A deficiency is the most important preventable cause of blindness. The earliest sign of deficiency is a loss of sensitivity to green light, followed by impairment to adapt to dim light, followed by night blindness. More prolonged deficiency leads to xerophthalmia: keratinization of the cornea and blindness. Vitamin A also has an important role in differentiation of immune system cells, and even mild deficiency leads to increased susceptibility to infectious diseases. Also, the synthesis of retinol binding protein is reduced in response to infection (it is a negative acute phase protein), decreasing the circulating concentration of the vitamin, and further impairing immune responses.
Vitamin A Is Toxic in Excess
There is only a limited capacity to metabolize vitamin A, and excessive intakes lead to accumulation beyond the capacity of binding proteins, so that unbound vitamin A causes tissue damage. Symptoms of toxicity affect the central nervous system (headache, nausea, ataxia, and anorexia, all associated with increased cerebrospinal fluid pressure); the liver (hepatomegaly with histologic changes and hyperlipidemia); calcium homeostasis (thickening of the long bones, hypercalcemia, and calcification of soft tissues); and the skin (excessive dryness, desquamation, and alopecia).
VITAMIN D IS REALLY A HORMONE
Vitamin D is not strictly a vitamin since it can be synthesized in the skin, and under most conditions that is the major source of the vitamin. Only when sunlight exposure is inadequate is a dietary source required. Its main function is in the regulation of calcium absorption and homeostasis; most of its actions are mediated by way of nuclear receptors that regulate gene expression. It also has a role in regulating cell proliferation and differentiation. There is evidence that intakes considerably higher than are required to maintain calcium homeostasis reduce the risk of insulin resistance, obesity and the metabolic syndrome, as well as various cancers. Deficiency, leading to rickets in children and osteomalacia in adults, continues to be a problem in northern latitudes, where sunlight exposure is inadequate.
Vitamin D Is Synthesized in the Skin
7-Dehydrocholesterol (an intermediate in the synthesis of cholesterol that accumulates in the skin) undergoes a nonenzymic reaction on exposure to ultraviolet light, yielding previtamin D (Figure 44–3). This undergoes a further reaction over a period of hours to form cholecalciferol, which is absorbed into the bloodstream. In temperate climates, the plasma concentration of vitamin D is highest at the end of summer and lowest at the end of winter. Beyond latitudes about 40° north or south, there is very little ultraviolet radiation of the appropriate wavelength in winter.
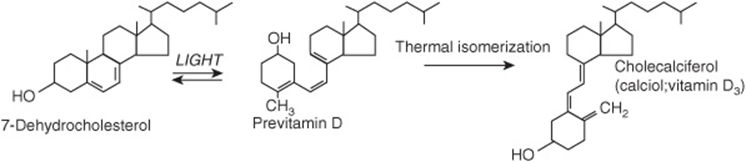
FIGURE 44–3 The synthesis of vitamin D in the skin.
Vitamin D Is Metabolized to the Active Metabolite, Calcitriol, in Liver & Kidney
Cholecalciferol, either synthesized in the skin or from food, undergoes two hydroxylations to yield the active metabolite, 1,25-dihydroxyvitamin D or calcitriol (Figure 44–4). Ergocalciferol from fortified foods undergoes similar hydroxylation to yield ercalcitriol. In the liver, cholecalciferol is hydroxylated to form the 25-hydroxy-derivative, calcidiol. This is released into the circulation bound to a vitamin D binding globulin, which is the main storage form of the vitamin. In the kidney, calcidiol undergoes either 1-hydroxylation to yield the active metabolite 1,25-dihydroxyvitamin D (calcitriol), or 24-hydroxylation to yield a probably inactive metabolite, 24,25-dihydroxyvitamin D (24-hydroxycalcidiol).
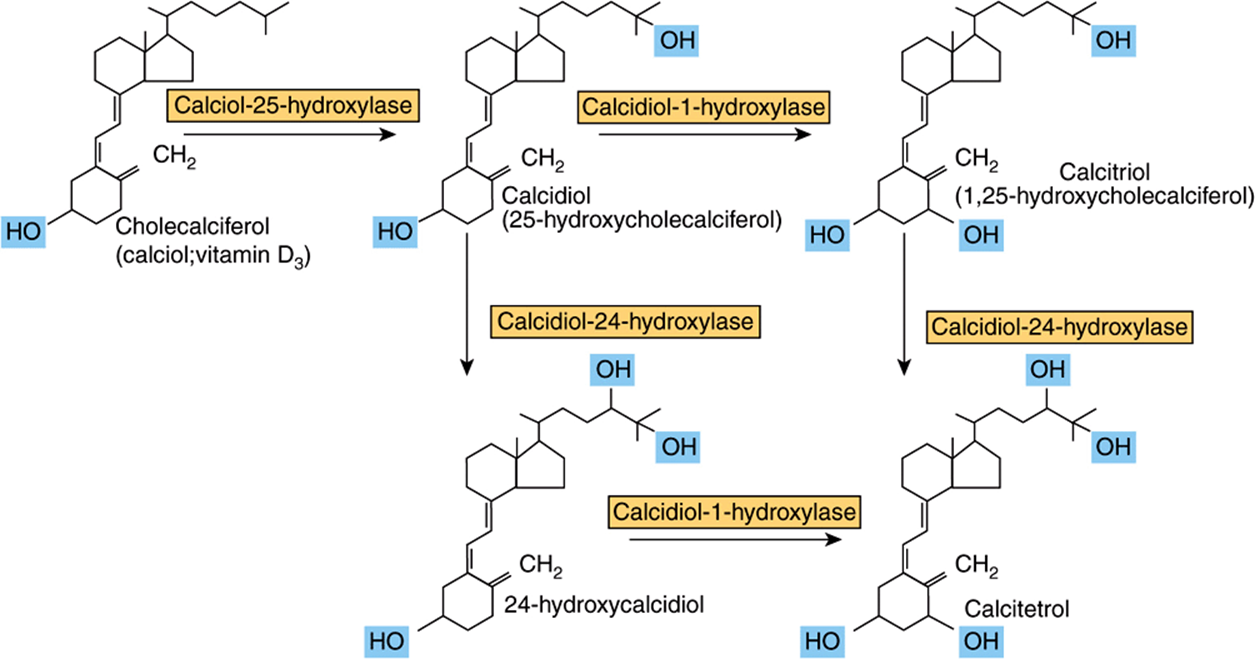
FIGURE 44–4 Metabolism of vitamin D.
Vitamin D Metabolism Is Both Regulated by and Regulates Calcium Homeostasis
The main function of vitamin D is in the control of calcium homeostasis, and in turn, vitamin D metabolism is regulated by factors that respond to plasma concentrations of calcium and phosphate. Calcitriol acts to reduce its own synthesis by inducing the 24-hydroxylase and repressing the 1-hydroxylase in the kidney. The principal function of vitamin D is to maintain the plasma calcium concentration. Calcitriol achieves this in three ways: it increases intestinal absorption of calcium; it reduces excretion of calcium (by stimulating resorption in the distal renal tubules); and it mobilizes bone mineral. In addition, calcitriol is involved in insulin secretion, synthesis and secretion of parathyroid and thyroid hormones, inhibition of production of interleukin by activated T-lymphocytes and of immunoglobulin by activated B-lymphocytes, differentiation of monocyte precursor cells, and modulation of cell proliferation. In most of these actions, it acts like a steroid hormone, binding to nuclear receptors and enhancing gene expression, although it also has rapid effects on calcium transporters in the intestinal mucosa. For further details of the role of calcitriol in calcium homeostasis, see Chapter 47.
Higher Intakes of Vitamin D may be Beneficial
There is growing evidence that higher vitamin D status is protective against various cancers, including prostate and colorectal cancer, and also against prediabetes and the metabolic syndrome. Desirable levels of intake may be considerably higher than current reference intakes, and could certainly not be met from unfortified foods. While increased sunlight exposure would meet the need, it carries the risk of developing skin cancer.
Vitamin D Deficiency Affects Children & Adults
In the vitamin D deficiency disease rickets, the bones of children are undermineralized as a result of poor absorption of calcium. Similar problems occur as a result of deficiency during the adolescent growth spurt. Osteomalacia in adults results from the demineralization of bone, especially in women who have little exposure to sunlight, especially after several pregnancies. Although vitamin D is essential for prevention and treatment of osteomalacia in the elderly, there is little evidence that it is beneficial in treating osteoporosis.
Vitamin D Is Toxic in Excess
Some infants are sensitive to intakes of vitamin D as low as 50 μg/day, resulting in an elevated plasma concentration of calcium. This can lead to contraction of blood vessels, high blood pressure, and calcinosis—the calcification of soft tissues. Although excess dietary vitamin D is toxic, excessive exposure to sunlight does not lead to vitamin D poisoning, because there is a limited capacity to form the precursor, 7-dehydrocholesterol, and prolonged exposure of previtamin D to sunlight leads to formation of inactive compounds.
VITAMIN E DOES NOT HAVE A PRECISELY DEFINED METABOLIC FUNCTION
No unequivocal unique function for vitamin E has been defined. It acts as a lipid-soluble antioxidant in cell membranes, where many of its functions can be provided by synthetic antioxidants, and is important in maintaining the fluidity of cell membranes. It also has a (relatively poorly defined) role in cell signaling. Vitamin E is the generic descriptor for two families of compounds, the tocopherols and the tocotrienols (Figure 44–5). The different vitamers have different biologic potency; the most active is D-α-tocopherol, and it is usual to express vitamin E intake in terms of milligrams D-α-tocopherol equivalents. Synthetic DL-a-tocopherol does not have the same biologic potency as the naturally occurring compound.
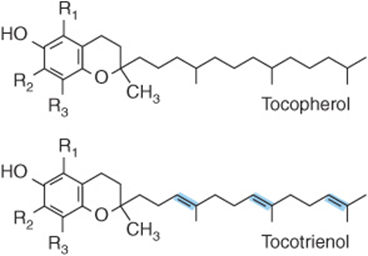
FIGURE 44–5 Vitamin E vitamers. In α-tocopherol and tocotrienol R1, R2, and R3 are all—CH3 groups. In the β-vitamers R2 is H, in the γ-vitamers R1 is H, and in the δ-vitamers R1 and R2 are both H.
Vitamin E Is the Major Lipid-Soluble Antioxidant in Cell Membranes and Plasma Lipoproteins
The main function of vitamin E is as a chain-breaking, free-radical-trapping antioxidant in cell membranes and plasma lipoproteins by reacting with the lipid peroxide radicals formed by peroxidation of polyunsaturated fatty acids (Chapter 45). The tocopheroxyl radical product is relatively unreactive, and ultimately forms nonradical compounds. Commonly, the tocopheroxyl radical is reduced back to tocopherol by reaction with vitamin C from plasma (Figure 44–6). The resultant monodehydroascorbate radical then undergoes enzymic or nonenzymic reaction to yield ascorbate and dehydroascorbate, neither of which is a radical.
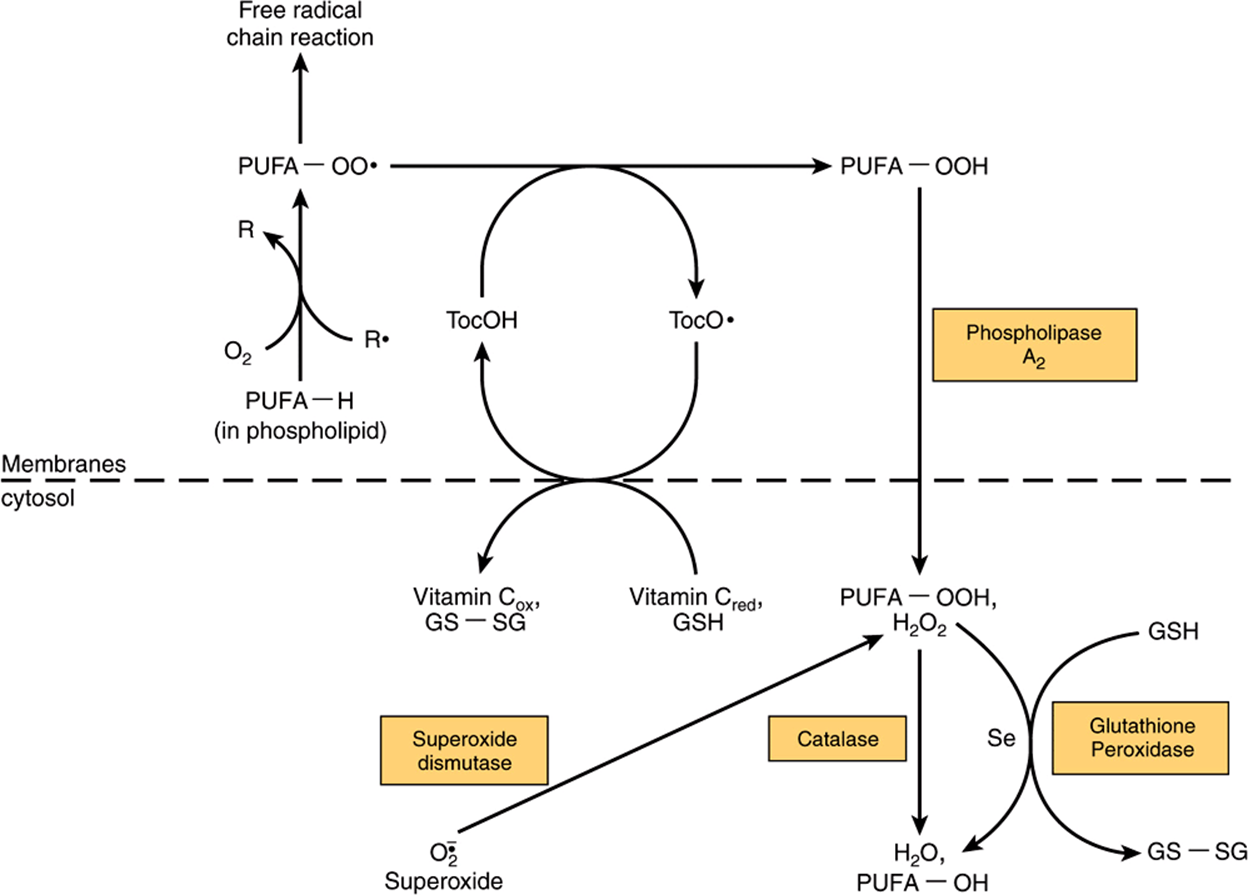
FIGURE 44–6 Interaction between antioxidants in the lipid phase (cell membranes) and the aqueous phase (cytosol). (R•, free radical; PUFA-OO•, peroxyl radical of polyunsaturated fatty acid in membrane phospholipid; PUFA-OOH, hydroxyperoxy polyunsaturated fatty acid in membrane phospholipid, released into the cytosol as hydroxyperoxy polyunsaturated fatty acid by the action of phospholipase A2; PUFA-OH, hydroxy polyunsaturated fatty acid; Toc-OH vitamin E [α-tocopherol]; TocO•, tocopheroxyl radical; Se, selenium; GSH, reduced glutathione; GS-SG, oxidized glutathione, which is reduced to GSH after reaction with NADPH, catalyzed by glutathione reductase; PUFA-H, polyunsaturated fatty acid.)
Vitamin E Deficiency
In experimental animals, vitamin E deficiency results in resorption of fetuses and testicular atrophy. Dietary deficiency of vitamin E in humans is unknown, although patients with severe fat malabsorption, cystic fibrosis, and some forms of chronic liver disease suffer deficiency because they are unable to absorb the vitamin or transport it, exhibiting nerve and muscle membrane damage. Premature infants are born with inadequate reserves of the vitamin. The erythrocyte membranes are abnormally fragile as a result of lipid peroxidation, leading to hemolytic anemia.
VITAMIN K IS REQUIRED FOR SYNTHESIS OF BLOOD-CLOTTING PROTEINS
Vitamin K was discovered as a result of investigations into the cause of a bleeding disorder, hemorrhagic (sweet clover) disease of cattle and of chickens fed on a fat-free diet. The missing factor in the diet of the chickens was vitamin K, while the cattle feed contained dicumarol, an antagonist of the vitamin. Antagonists of vitamin K are used to reduce blood coagulation in patients at risk of thrombosis; the most widely used is warfarin.
Three compounds have the biological activity of vitamin K (Figure 44–7): phylloquinone, the normal dietary source, found in green vegetables; menaquinones, synthesized by intestinal bacteria, with differing lengths of side chain; and menadione and menadiol diacetate, synthetic compounds that can be metabolized to phylloquinone. Menaquinones are absorbed to some extent, but it is not clear to what extent they are biologically active as it is possible to induce signs of vitamin K deficiency simply by feeding a phylloquinone-deficient diet, without inhibiting intestinal bacterial action.
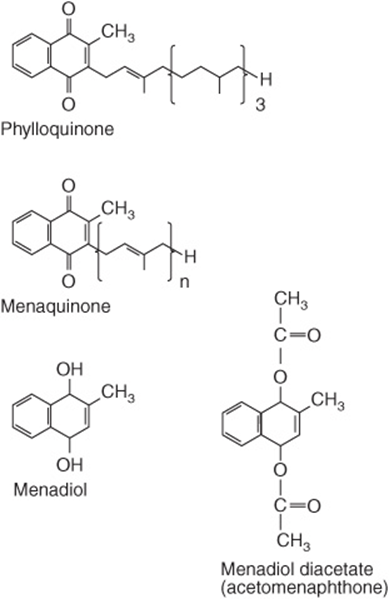
FIGURE 44–7 The vitamin K vitamers. Menadiol (or menadione) and menadiol diacetate are synthetic compounds that are converted to menaquinone in the liver.
Vitamin K Is the Coenzyme for Carboxylation of Glutamate in Postsynthetic Modification of Calcium-Binding Proteins
Vitamin K is the cofactor for the carboxylation of glutamate residues in the postsynthetic modification of proteins to form the unusual amino acid γ-carboxyglutamate (Gla) (Figure 44–8). Initially, vitamin K hydroquinone is oxidized to the epoxide, which activates a glutamate residue in the protein substrate to a carbanion, which reacts nonenzymically with carbon dioxide to form γ-carboxyglutamate. Vitamin K epoxide is reduced to the quinone by a warfarin-sensitive reductase, and the quinone is reduced to the active hydroquinone by either the same warfarin-sensitive reductase or a warfarin-insensitive quinone reductase. In the presence of warfarin, vitamin K epoxide cannot be reduced, but accumulates and is excreted. If enough vitamin K (as the quinone) is provided in the diet, it can be reduced to the active hydroquinone by the warfarin-insensitive enzyme, and carboxylation can continue, with stoichiometric utilization of vitamin K and excretion of the epoxide. A high dose of vitamin K is the antidote to an overdose of warfarin.
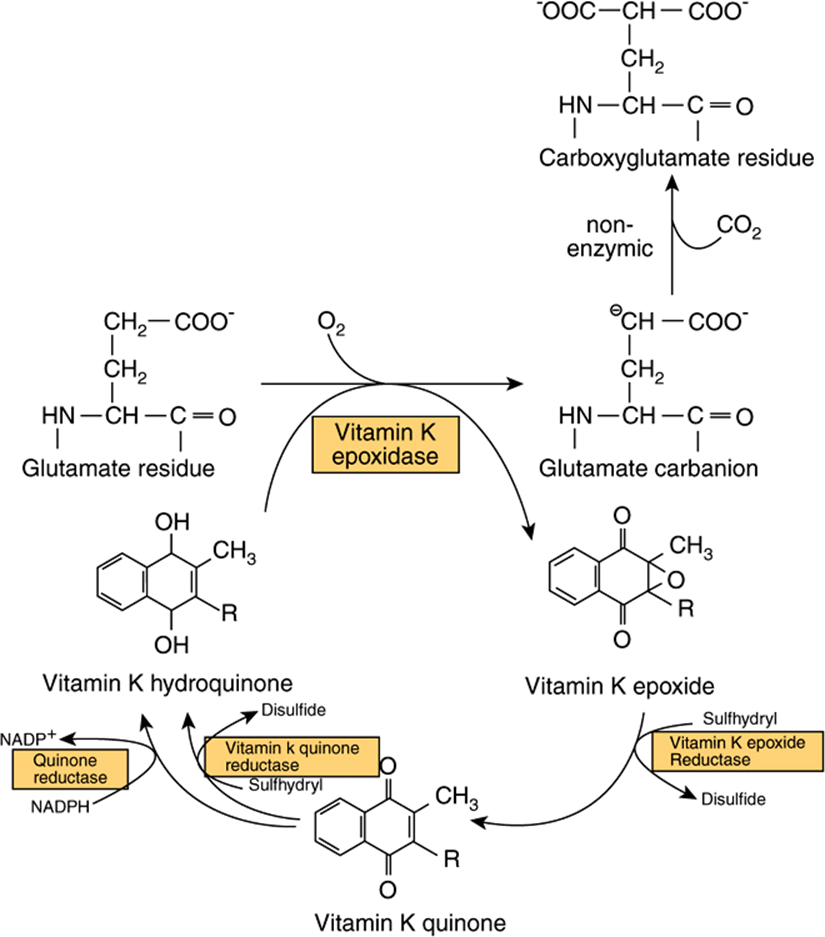
FIGURE 44–8 The role of vitamin K in the synthesis of γ-carboxyglutamate.
Prothrombin and several other proteins of the blood-clotting system (Factors VII, IX, and X, and proteins C and S, Chapter 50) each contain 4-6 γ-carboxyglutamate residues. γ-Carboxyglutamate chelates calcium ions, and so permits the binding of the blood-clotting proteins to membranes. In vitamin K deficiency, or in the presence of warfarin, an abnormal precursor of prothrombin (preprothrombin) containing little or no γ-carboxyglutamate, and incapable of chelating calcium, is released into the circulation.
Vitamin K Is Also Important in Synthesis of Bone and other Calcium-Binding Proteins
A number of other proteins undergo the same vitamin K-dependent carboxylation of glutamate to γ-carboxyglutamate, including osteocalcin and the matrix Gla protein in bone, nephrocalcin in kidney and the product of the growth arrest specific gene Gas6, which is involved in both the regulation of differentiation and development in the nervous system, and control of apoptosis in other tissues. All of these γ-carboxyglutamate containing proteins bind calcium, which causes a conformational change so that they interact with membrane phospholipids. The release into the circulation of osteocalcin provides an index of vitamin D status.
WATER-SOLUBLE VITAMINS
VITAMIN B1 (THIAMIN) HAS A KEY ROLE IN CARBOHYDRATE METABOLISM
Thiamin has a central role in energy-yielding metabolism, and especially the metabolism of carbohydrates (Figure 44–9). Thiamin diphosphate is the coenzyme for three multienzyme complexes that catalyze oxidative decarboxylation reactions: pyruvate dehydrogenase in carbohydrate metabolism (Chapter 17); α-ketoglutarate dehydrogenase in the citric acid cycle (Chapter 17); and the branched-chain keto-acid dehydrogenase involved in the metabolism of leucine, isoleucine, and valine (Chapter 29). In each case, the thiamin diphosphate provides a reactive carbon on the thiazole moiety that forms a carbanion, which then adds to the carbonyl group, eg, pyruvate. The addition compound is then decarboxylated, eliminating CO2. Thiamin diphosphate is also the coenzyme for transketolase, in the pentose phosphate pathway (Chapter 21).
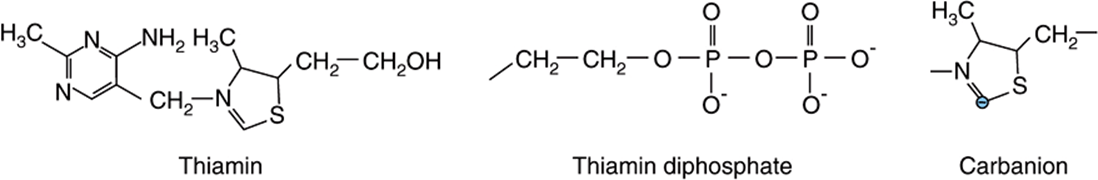
FIGURE 44–9 Thiamin, thiamin diphosphate, and the carbanion form.
Thiamin triphosphate has a role in nerve conduction; it phosphorylates, and so activates, a chloride channel in the nerve membrane.
Thiamin Deficiency Affects the Nervous System & the Heart
Thiamin deficiency can result in three distinct syndromes: a chronic peripheral neuritis, beriberi, which may or may not be associated with heart failure and edema; acute pernicious (fulminating) beriberi (shoshin beriberi), in which heart failure and metabolic abnormalities predominate, without peripheral neuritis; and Wernicke encephalopathy with Korsakoff psychosis, which is associated especially with alcohol and narcotic abuse. The role of thiamin diphosphate in pyruvate dehydrogenase means that in deficiency there is impaired conversion of pyruvate to acetyl CoA. In subjects on a relatively high carbohydrate diet, this results in increased plasma concentrations of lactate and pyruvate, which may cause life-threatening lactic acidosis.
Thiamin Nutritional Status Can Be Assessed by Erythrocyte Transketolase Activation
The activation of apo-transketolase (the enzyme protein) in erythrocyte lysate by thiamin diphosphate added in vitro has become the accepted index of thiamin nutritional status.
VITAMIN B2 (RIBOFLAVIN) HAS A CENTRAL ROLE IN ENERGY-YIELDING METABOLISM
Riboflavin provides the reactive moieties of the coenzymes flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD) (Figure 44–10). FMN is formed by ATP-dependent phosphorylation of riboflavin, whereas FAD is synthesized by further reaction with ATP in which its AMP moiety is transferred to FMN. The main dietary sources of riboflavin are milk and dairy products. In addition, because of its intense yellow color, riboflavin is widely used as a food additive.
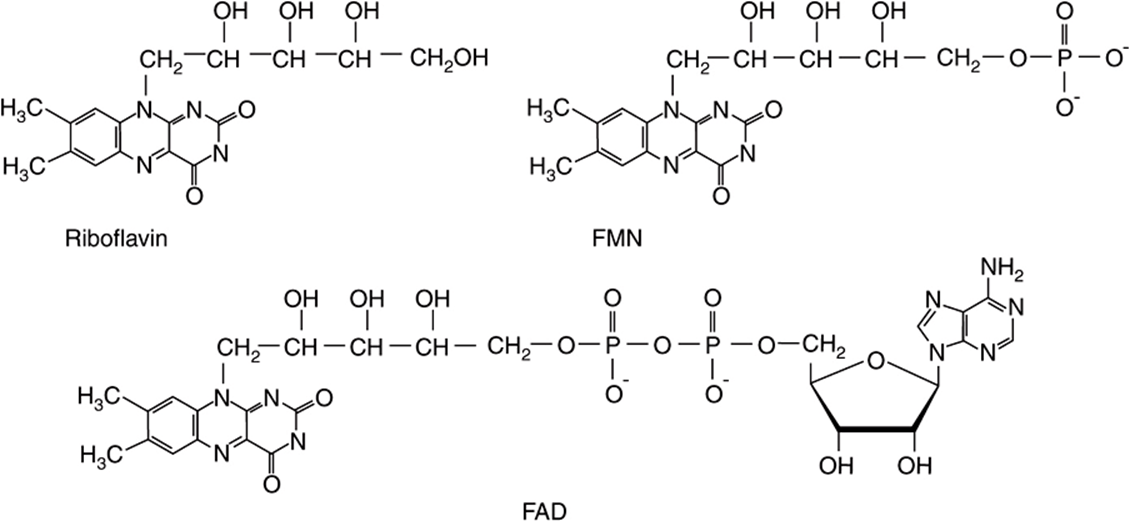
FIGURE 44–10 Riboflavin and the coenzymes flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD).
Flavin Coenzymes Are Electron Carriers in Oxidoreduction Reactions
These include the mitochondrial respiratory chain, key enzymes in fatty acid and amino acid oxidation, and the citric acid cycle. Reoxidation of the reduced flavin in oxygenases and mixed-function oxidases proceeds by way of formation of the flavin radical and flavin hydroperoxide, with the intermediate generation of superoxide and perhydroxyl radicals and hydrogen peroxide. Because of this, flavin oxidases make a significant contribution to the total oxidant stress in the body (Chapter 45).
Riboflavin Deficiency Is Widespread but Not Fatal
Although riboflavin is centrally involved in lipid and carbohydrate metabolism, and deficiency occurs in many countries, it is not fatal, because there is very efficient conservation of tissue riboflavin. Riboflavin released by the catabolism of enzymes is rapidly incorporated into newly synthesized enzymes. Deficiency is characterized by cheilosis, desquamation and inflammation of the tongue, and a seborrheic dermatitis. Riboflavin nutritional status is assessed by measurement of the activation of erythrocyte glutathione reductase by FAD added in vitro.
NIACIN IS NOT STRICTLY A VITAMIN
Niacin was discovered as a nutrient during studies of pellagra. It is not strictly a vitamin since it can be synthesized in the body from the essential amino acid tryptophan. Two compounds, nicotinic acid and nicotinamide, have the biologic activity of niacin; its metabolic function is as the nicotinamide ring of the coenzymes NAD and NADP in oxidation/reduction reactions (Figure 44–11). Some 60 mg of tryptophan is equivalent to 1 mg of dietary niacin. The niacin content of foods is expressed as
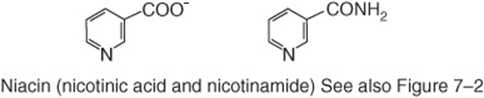
FIGURE 44–11 Niacin (nicotinic acid and nicotinamide).
![]()
Since most of the niacin in cereals is biologically unavailable, this is discounted.
NAD Is the Source of ADP-Ribose
In addition to its coenzyme role, NAD is the source of ADP-ribose for the ADP-ribosylation of proteins and polyADP-ribosylation of nucleoproteins involved in the DNA repair mechanism. Cyclic ADP-ribose and nicotinic acid adenine dinucleotide, formed from NAD, act to increase intracellular calcium in response to neurotransmitters and hormones.
Pellagra Is Caused by Deficiency of Tryptophan & Niacin
Pellagra is characterized by a photosensitive dermatitis. As the condition progresses, there is dementia and possibly diarrhea. Untreated pellagra is fatal. Although the nutritional etiology of pellagra is well established, and tryptophan or niacin prevents or cures the disease, additional factors, including deficiency of riboflavin or vitamin B6, both of which are required for synthesis of nicotinamide from tryptophan, may be important. In most outbreaks of pellagra, twice as many women as men are affected, probably the result of inhibition of tryptophan metabolism by estrogen metabolites.
Pellagra Can Occur as a Result of Disease Despite an Adequate Intake of Tryptophan & Niacin
A number of genetic diseases that result in defects of tryptophan metabolism are associated with the development of pellagra, despite an apparently adequate intake of both tryptophan and niacin. Hartnup disease is a rare genetic condition in which there is a defect of the membrane transport mechanism for tryptophan, resulting in large losses as a result of intestinal malabsorption and failure of the renal reabsorption mechanism. In carcinoid syndrome, there is metastasis of a primary liver tumor of enterochromaffin cells, which synthesize 5-hydroxytryptamine. Overproduction of 5-hydroxytryptamine may account for as much as 60% of the body’s tryptophan metabolism, causing pellagra because of the diversion away from NAD synthesis.
Niacin Is Toxic in Excess
Nicotinic acid has been used to treat hyperlipidemia when of the order of 1-6 g/day are required, causing dilatation of blood vessels and flushing, along with skin irritation. Intakes of both nicotinic acid and nicotinamide in excess of 500 mg/day also cause liver damage.
VITAMIN B6 IS IMPORTANT IN AMINO ACID & GLYCOGEN METABOLISM & IN STEROID HORMONE ACTION
Six compounds have vitamin B6 activity (Figure 44–12): pyri-doxine, pyridoxal, pyridoxamine, and their 5′-phosphates. The active coenzyme is pyridoxal 5′-phosphate. Some 80% of the body’s total vitamin B6 is pyridoxal phosphate in muscle, mostly associated with glycogen phosphorylase. This is not available in deficiency, but is released in starvation, when glycogen reserves become depleted, and is then available, especially in liver and kidney, to meet increased requirement for gluconeogenesis from amino acids.
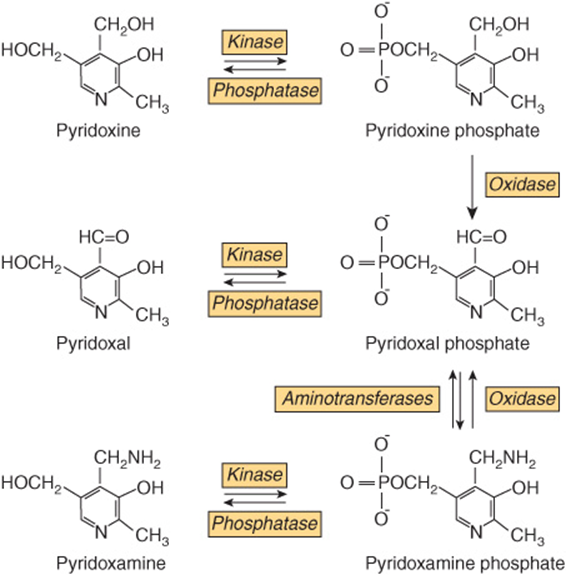
FIGURE 44–12 Interconversion of the vitamin B6 vitamers.
Vitamin Be Has Several Roles in Metabolism
Pyridoxal phosphate is a coenzyme for many enzymes involved in amino acid metabolism, especially transamination and decarboxylation. It is also the cofactor of glycogen phosphorylase, where the phosphate group is catalytically important. In addition, B6 is important in steroid hormone action. Pyridoxal phosphate removes the hormone-receptor complex from DNA binding, terminating the action of the hormones. In vitamin B6 deficiency, there is increased sensitivity to the actions of low concentrations of estrogens, androgens, cortisol, and vitamin D.
Vitamin B6 Deficiency Is Rare
Although clinical deficiency disease is rare, there is evidence that a significant proportion of the population have marginal vitamin B6 status. Moderate deficiency results in abnormalities of tryptophan and methionine metabolism. Increased sensitivity to steroid hormone action may be important in the development of hormone-dependent cancer of the breast, uterus, and prostate, and vitamin B6 status may affect the prognosis.
Vitamin B6 Status Is Assessed by Assaying Erythrocyte Transaminases
The most widely used method of assessing vitamin B6 status is by the activation of erythrocyte transaminases by pyridoxal phosphate added in vitro, expressed as the activation coefficient.
In Excess, Vitamin B6 Causes Sensory Neuropathy
The development of sensory neuropathy has been reported in patients taking 2-7 g of pyridoxine per day for a variety of reasons (there is some slight evidence that it is effective in treating premenstrual syndrome). There was some residual damage after withdrawal of these high doses; other reports suggest that intakes in excess of 200 mg/d are associated with neurologic damage.
VITAMIN B12 IS FOUND ONLY IN FOODS OF ANIMAL ORIGIN
The term “vitamin B12” is used as a generic descriptor for the cobalamins—those corrinoids (cobalt-containing compounds possessing the corrin ring) having the biologic activity of the vitamin (Figure 44–13). Some corrinoids that are growth factors for micro-organisms not only have no vitamin B12 activity, but may also be antimetabolites of the vitamin. Although it is synthesized exclusively by microorganisms, for practical purposes vitamin B12 is found only in foods of animal origin, there being no plant sources of this vitamin. This means that strict vegetarians (vegans) are at risk of developing B deficiency. The small amounts of the vitamin formed by bacteria on the surface of fruits may be adequate to meet requirements, but preparations of vitamin B12 made by bacterial fermentation are available.
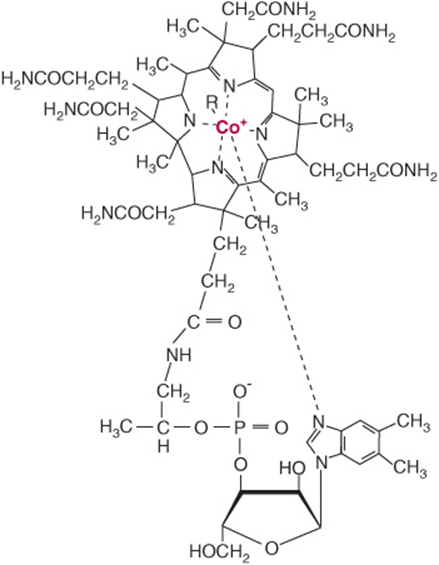
FIGURE 44–13 Vitamin B12. Four coordination sites on the central cobalt atom are chelated by the nitrogen atoms of the corrin ring, and one by the nitrogen of the dimethylbenzimidazole nucleotide. The sixth coordination site may be occupied by: CN– (cyanocobalamin), OH– (hydroxocobalamin), H2O (aquocobalamin,—CH3 (methyl cobalamin), or 5′-deoxyadenosine (adenosylcobalamin).
Vitamin B12 Absorption Requires Two Binding Proteins
Vitamin B12 is absorbed bound to intrinsic factor, a small glycoprotein secreted by the parietal cells of the gastric mucosa. Gastric acid and pepsin release the vitamin from protein binding in food and make it available to bind to cobalophilin, a binding protein secreted in the saliva. In the duodenum, cobalophilin is hydrolyzed, releasing the vitamin for binding to intrinsic factor. Pancreatic insufficiency can therefore be a factor in the development of vitamin B12 deficiency, resulting in the excretion of cobalophilin-bound vitamin B12. Intrinsic factor binds only the active vitamin B12 vitamers and not other corrinoids. Vitamin B12 is absorbed from the distal third of the ileum via receptors that bind the intrinsic factor-vitamin B12 complex, but not free intrinsic factor or free vitamin.
There Are Three Vitamin B12-Dependent Enzymes
Methylmalonyl CoA mutase, leucine aminomutase, and methionine synthase (Figure 44–14) are vitamin B12-dependent enzymes. Methylmalonyl CoA is formed as an intermediate in the catabolism of valine and by the carboxylation of propionyl CoA arising in the catabolism of isoleucine, cholesterol, and, rarely, fatty acids with an odd number of carbon atoms or directly from propionate, a major product of microbial fermentation in the rumen. It undergoes a vitamin B12-dependent rearrangement to succinyl CoA, catalyzed by methylmalonyl CoA mutase (Figure 20–2). The activity of this enzyme is greatly reduced in vitamin B12 deficiency, leading to an accumulation of methylmalonyl CoA and urinary excretion of methylmalonic acid, which provides a means of assessing vitamin B12 nutritional status.
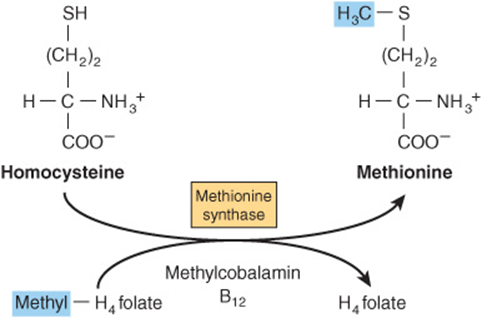
FIGURE 44–14 Homocysteine and the “folate trap.” Vitamin B12 deficiency leads to impairment of methionine synthase, resulting in accumulation of homocysteine and trapping folate as methyltetrahyd rofolate.
Vitamin B12 Deficiency Causes Pernicious Anemia
Pernicious anemia arises when vitamin B12 deficiency impairs the metabolism of folic acid, leading to functional folate deficiency that disturbs erythropoiesis, causing immature precursors of erythrocytes to be released into the circulation (megaloblastic anemia). The most common cause of pernicious anemia is failure of the absorption of vitamin B12 rather than dietary deficiency. This can be the result of failure of intrinsic factor secretion caused by autoimmune disease affecting parietal cells or from production of anti-intrinsic factor antibodies. There is irreversible degeneration of the spinal cord in pernicious anemia, as a result of failure of methylation of one arginine residue in myelin basic protein. This is the result of methionine deficiency in the central nervous system, rather than secondary folate deficiency.
THERE ARE MULTIPLE FORMS OF FOLATE IN THE DIET
The active form of folic acid (pteroyl glutamate) is tetrahydrofolate (Figure 44–15). The folates in foods may have up to seven additional glutamate residues linked by γ-peptide bonds. In addition, all of the one-carbon substituted folates in Figure 44–15 may also be present in foods. The extent to which the different forms of folate can be absorbed varies, and folate intakes are calculated as dietary folate equivalents—the sum of μg food folates + 1.7 × μg of folic acid (used in food enrichment).
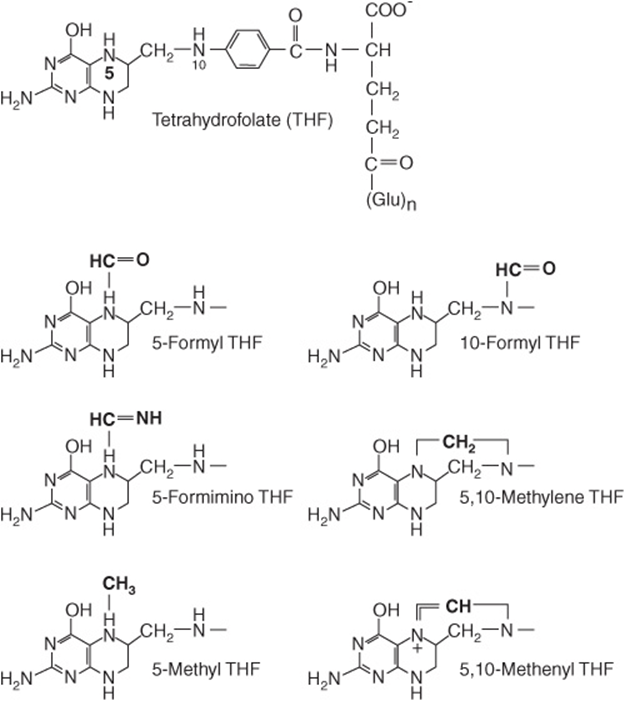
FIGURE 44–15 Tetrahydrofolic acid and the one-carbon substituted folates.
Tetrahydrofolate Is a Carrier of One-Carbon Units
Tetrahydrofolate can carry one-carbon fragments attached to N-5 (formyl, formimino, or methyl groups), N-10 (formyl) or bridging N-5-N-10 (methylene or methenyl groups). 5-Formyl-tetrahydrofolate is more stable than folate and is therefore used pharmaceutically (known as folinic acid), and the synthetic (racemic) compound (leucovorin). The major point of entry for one-carbon fragments into substituted folates is methylene-tetrahydrofolate (Figure 44–16), which is formed by the reaction of glycine, serine, and choline with tetrahydrofolate. Serine is the most important source of substituted folates for biosynthetic reactions, and the activity of serine hydroxymethyltransferase is regulated by the state of folate substitution and the availability of folate. The reaction is reversible, and in liver it can form serine from glycine as a substrate for gluconeogenesis. Methylene-, methenyl-, and 10-formyl-tetrahydrofolates are interconvertible. When one-carbon folates are not required, the oxidation of formyl-tetrahydrofolate to yield carbon dioxide provides a means of maintaining a pool of free folate.
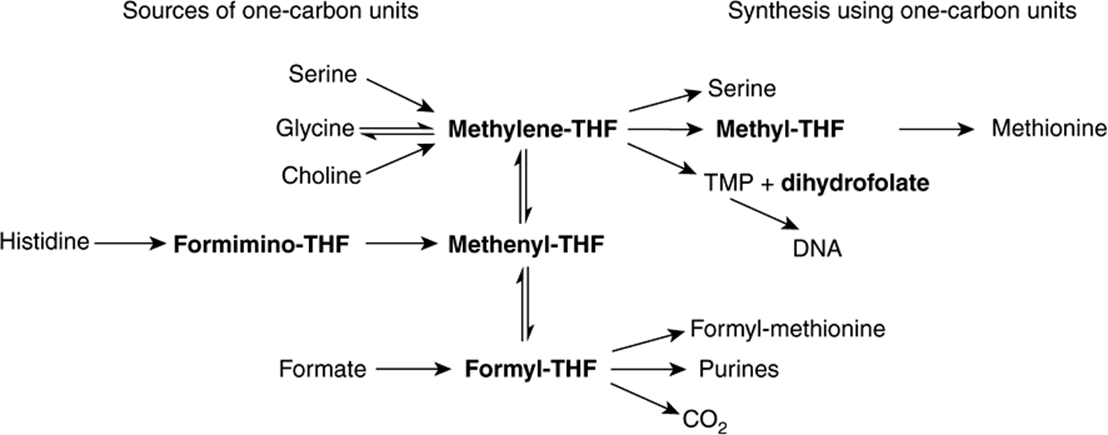
FIGURE 44–16 Sources and utilization of one-carbon substituted folates.
Inhibitors of Folate Metabolism Provide Cancer Chemotherapy, Antibacterial, & Antimalarial Drugs
The methylation of deoxyuridine monophosphate (dUMP) to thymidine monophosphate (TMP), catalyzed by thymidylate synthase, is essential for the synthesis of DNA. The one-carbon fragment of methylene-tetrahydrofolate is reduced to a methyl group with release of dihydrofolate, which is then reduced back to tetrahydrofolate by dihydrofolate reductase. Thymidylate synthase and dihydrofolate reductase are especially active in tissues with a high rate of cell division. Methotrexate, an analog of 10-methyl-tetrahydrofolate, inhibits dihydrofolate reductase and has been exploited as an anticancer drug. The dihydrofolate reductases of some bacteria and parasites differ from the human enzyme; inhibitors of these enzymes can be used as antibacterial drugs (eg, trimethoprim) and antimalarial drugs (eg, pyrimethamine).
Vitamin B12 Deficiency Causes Functional Folate Deficiency—the “Folate Trap”
When acting as a methyl donor, S-adenosyl methionine forms homocysteine, which may be remethylated by methyl-tetrahydrofolate catalyzed by methionine synthase, a vitamin B12-dependent enzyme (Figure 44–14). As the reduction of methylene-tetrahydrofolate to methyl-tetrahydrofolate is irreversible and the major source of tetrahydrofolate for tissues is methyltetrahydrofolate, the role of methionine synthase is vital, and provides a link between the functions of folate and vitamin B12 Impairment of methionine synthase in vitamin B12 deficiency results in the accumulation of methyltetrahydrofolate—the “folate trap.” There is therefore functional deficiency of folate, secondary to the deficiency of vitamin B12.
Folate Deficiency Causes Megaloblastic Anemia
Deficiency of folic acid itself or deficiency of vitamin B12, which leads to functional folic acid deficiency, affects cells that are dividing rapidly because they have a large requirement for thymidine for DNA synthesis. Clinically, this affects the bone marrow, leading to megaloblastic anemia.
Folic Acid Supplements Reduce the Risk of Neural Tube Defects & Hyperhomocysteinemia, & May Reduce the Incidence of Cardiovascular Disease & Some Cancers
Supplements of 400 μg/day of folate begun before conception result in a significant reduction in the incidence of spina bifida and other neural tube defects. Because of this, there is mandatory enrichment of flour with folic acid in many countries. Elevated blood homocysteine is a significant risk factor for atherosclerosis, thrombosis, and hypertension. The condition is the result of an impaired ability to form methyltetrahydrofolate by methylene-tetrahydrofolate reductase, causing functional folate deficiency, resulting in failure to remethylate homocysteine to methionine. People with an abnormal variant of methylene-tetrahydrofolate reductase that occurs in 5-10% of the population do not develop hyperhomocysteinemia if they have a relatively high intake of folate. A number of placebo-controlled trials of supplements of folate (commonly together with vitamins B6 and B12) have shown the expected lowering of plasma homocysteine, but apart from reduced incidence of stroke there has been no effect on cardiovascular disease.
There is also evidence that low folate status results in impaired methylation of CpG islands in DNA, which is a factor in the development of colorectal and other cancers. A number of studies suggest that folate supplementation or food enrichment may reduce the risk of developing some cancers. However, there is some evidence that folate supplements increase the rate of transformation of preneoplastic colorectal polyps into cancers, so that people with such polyps are at increased risk of developing colorectal cancer if they have a high folate intake.
Folate Enrichment of Foods May Put Some People at Risk
Folate supplements will rectify the megaloblastic anemia of vitamin B12 deficiency but may hasten the development of the (irreversible) nerve damage found in vitamin B12 deficiency. There is also antagonism between folic acid and the anticonvulsants used in the treatment of epilepsy, and, as noted above, there is some evidence that folate supplements may increase the risk of developing colorectal cancer among people with preneoplastic colorectal polyps.
DIETARYBIOTIN DEFICIENCY IS UNKNOWN
The structures of biotin, biocytin, and carboxybiotin (the active metabolic intermediate) are shown in Figure 44–17. Biotin is widely distributed in many foods as biocytin (ε-amino-biotinyllysine), which is released on proteolysis. It is synthesized by intestinal flora in excess of requirements. Deficiency is unknown, except among people maintained for many months on total parenteral nutrition, and a very small number who eat abnormally large amounts of uncooked egg white, which contains avidin, a protein that binds biotin and renders it unavailable for absorption.
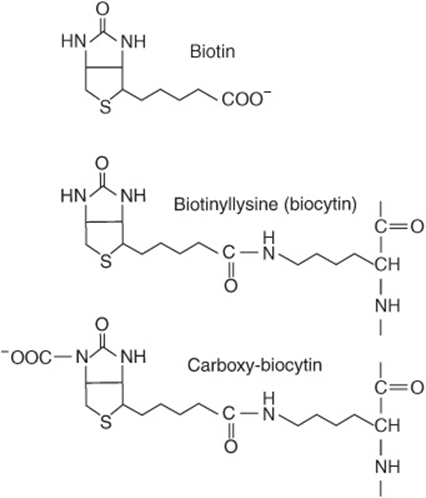
FIGURE 44–17 Biotin, biocytin, and carboxy-biocytin.
Biotin Is a Coenzyme of Carboxylase Enzymes
Biotin functions to transfer carbon dioxide in a small number of reactions: acetyl-CoA carboxylase (Figure 23–1), pyruvate carboxylase (Figure 20–1), propionyl-CoA carboxylase (Figure 20–2), and methylcrotonyl-CoA carboxylase. A holocarboxylase synthetase catalyzes the transfer of biotin onto a lysine residue of the apo-enzyme to form the biocytin residue of the holoenzyme. The reactive intermediate is 1-N-carboxybiocytin, formed from bicarbonate in an ATP-dependent reaction. The carboxy group is then transferred to the substrate for carboxylation.
Biotin also has a role in regulation of the cell cycle, acting to biotinylate key nuclear proteins.
AS PART OF CoA & ACP, PANTOTHENIC ACID ACTS AS A CARRIER OF ACYL GROUPS
Pantothenic acid has a central role in acyl group metabolism when acting as the pantetheine functional moiety of coenzyme A or acyl carrier protein (ACP) (Figure 44–18). The pantetheine moiety is formed after combination of pantothenate with cysteine, which provides the -SH prosthetic group of CoA and ACP. CoA takes part in reactions of the citric acid cycle (Chapter 17), fatty acid oxidation (Chapter 22), acetylations, and cholesterol synthesis (Chapter 26). ACP participates in fatty acid synthesis (Chapter 23). The vitamin is widely distributed in all food-stuffs, and deficiency has not been unequivocally reported in humans except in specific depletion studies.
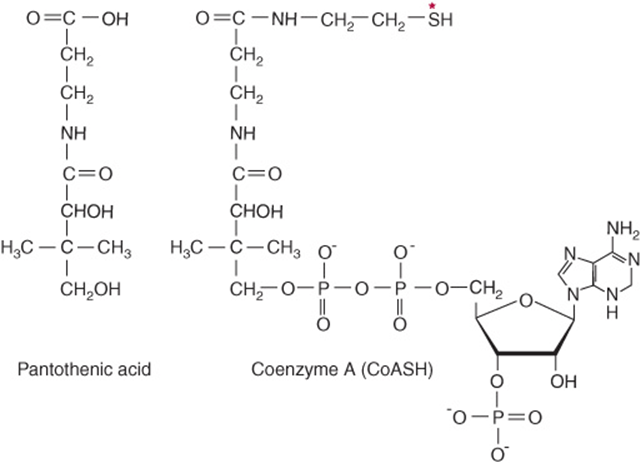
FIGURE 44–18 Pantothenic acid and coenzyme A. Asterisk shows site of acylation by fatty acids.
ASCORBIC ACID IS A VITAMIN FOR ONLY SOME SPECIES
Vitamin C (Figure 44–19) is a vitamin for human beings and other primates, the guinea pig, bats, passeriform birds, and most fishes and invertebrates; other animals synthesize it as an intermediate in the uronic acid pathway of glucose metabolism (Figure 21–4). In those species for which it is a vitamin, there is a block in the pathway as a result of the absence of gulonolactone oxidase. Both ascorbic acid and dehydroascorbic acid have vitamin activity.
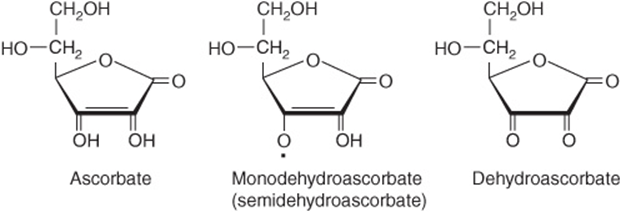
FIGURE 44–19 Vitamin C.
Vitamin C Is the Coenzyme for Two Groups of Hydroxylases
Ascorbic acid has specific roles in the copper-containing hydroxylases and the α-ketoglutarate-linked iron-containing hydroxylases. It also increases the activity of a number of other enzymes in vitro, although this is a nonspecific reducing action. In addition, it has a number of nonenzymic effects as a result of its action as a reducing agent and oxygen radical quencher (Chapter 45).
Dopamine β-hydroxylase is a copper-containing enzyme involved in the synthesis of the catecholamines (norepinephrine and epinephrine), from tyrosine in the adrenal medulla and central nervous system. During hydroxylation the Cu2+ is oxidized to Cu2+; reduction back to Cu+ specifically requires ascorbate, which is oxidized to monodehydroascorbate.
A number of peptide hormones have a carboxy terminal amide that is derived from a terminal glycine residue. This glycine is hydroxylated on the α-carbon by a copper-containing enzyme, peptidylglycine hydroxylase, which, again, requires ascorbate for reduction of Cu2+.
A number of iron-containing, ascorbate-requiring hydroxylases share a common reaction mechanism, in which hydroxylation of the substrate is linked to oxidative decarboxylation of α-ketoglutarate. Many of these enzymes are involved in the modification of precursor proteins. Proline and lysine hydroxylases are required for the postsynthetic modification of procollagen to collagen, and proline hydroxylase is also required in formation of osteocalcinand the C1q component of complement. Aspartate β-hydroxylase is required for the postsynthetic modification of the precursor of protein C, the vitamin K-dependent protease that hydrolyzes activated factor V in the blood-clotting cascade (Chapter 50). Trimethyllysine and γ-butyrobetaine hydroxylases are required for the synthesis of carnitine.
Vitamin C Deficiency Causes Scurvy
Signs of vitamin C deficiency include skin changes, fragility of blood capillaries, gum decay, tooth loss, and bone fracture, many of which can be attributed to deficient collagen synthesis.
There May Be Benefits from Higher Intakes of Vitamin C
At intakes above about 100 mg/day, the body’s capacity to metabolize vitamin C is saturated, and any further intake is excreted in the urine. However, in addition to its other roles, vitamin C enhances the absorption of inorganic iron, and this depends on the presence of the vitamin in the gut. Therefore, increased intakes may be beneficial. There is very little good evidence that high doses of vitamin C prevent the common cold, although they may reduce the duration and severity of symptoms.
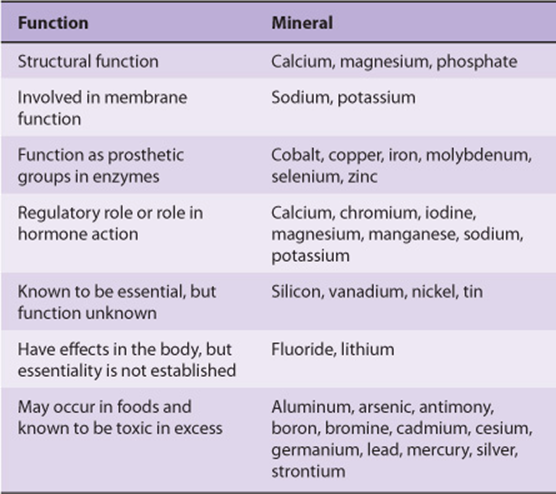
MINERALS ARE REQUIRED FOR BOTH PHYSIOLOGIC & BIOCHEMICAL FUNCTIONS
Many of the essential minerals (Table 44-6) are widely distributed in foods, and most people eating a mixed diet are likely to receive adequate intakes. The amounts required vary from grams per day for sodium and calcium, through milligrams per day (eg, iron and zinc), to micrograms per day for the trace elements. In general, mineral deficiencies occur when foods come from one region where the soil may be deficient in some minerals (eg, iodine and selenium, deficiencies of both of which occur in many areas of the world); when foods come from a variety of regions, mineral deficiency is less likely to occur. However, iron deficiency is a general problem, because if iron losses from the body are relatively high (eg, from heavy menstrual blood loss), it is difficult to achieve an adequate intake to replace losses. Foods grown on soil containing high levels of selenium cause toxicity, and excessive intakes of sodium cause hypertension in susceptible people.
TABLE 44–6 Classification of Minerals According to Their Function
SUMMARY
![]() Vitamins are organic nutrients with essential metabolic functions, generally required in small amounts in the diet because they cannot be synthesized by the body. The lipid-soluble vitamins (A, D, E, and K) are hydrophobic molecules requiring normal fat absorption for their absorption and the avoidance of deficiency.
Vitamins are organic nutrients with essential metabolic functions, generally required in small amounts in the diet because they cannot be synthesized by the body. The lipid-soluble vitamins (A, D, E, and K) are hydrophobic molecules requiring normal fat absorption for their absorption and the avoidance of deficiency.
![]() Vitamin A (retinol), present in meat, and the provitamin (β-carotene), found in plants, form retinaldehyde, utilized in vision, and retinoic acid, which acts in the control of gene expression.
Vitamin A (retinol), present in meat, and the provitamin (β-carotene), found in plants, form retinaldehyde, utilized in vision, and retinoic acid, which acts in the control of gene expression.
![]() Vitamin D is a steroid prohormone yielding the active hormone calcitriol, which regulates calcium and phosphate metabolism; deficiency leads to rickets and osteomalacia. It has a role in controlling cell differentiation and insulin secretion.
Vitamin D is a steroid prohormone yielding the active hormone calcitriol, which regulates calcium and phosphate metabolism; deficiency leads to rickets and osteomalacia. It has a role in controlling cell differentiation and insulin secretion.
![]() Vitamin E (tocopherol) is the most important lipid-soluble antioxidant in the body, acting in the lipid phase of membranes protecting against the effects of free radicals.
Vitamin E (tocopherol) is the most important lipid-soluble antioxidant in the body, acting in the lipid phase of membranes protecting against the effects of free radicals.
![]() Vitamin K functions acts as the cofactor of a carboxylase that acts on glutamate residues of precursor proteins of clotting factors and bone and other proteins to enable them to chelate calcium.
Vitamin K functions acts as the cofactor of a carboxylase that acts on glutamate residues of precursor proteins of clotting factors and bone and other proteins to enable them to chelate calcium.
![]() The water-soluble vitamins of the B complex act as enzyme cofactors. Thiamin is a cofactor in oxidative decarboxylation of α-keto acids and of transketolase in the pentose phosphate pathway. Riboflavin and niacin are important cofactors in oxidoreduction reactions, present in flavoprotein enzymes and in NAD and NADP, respectively.
The water-soluble vitamins of the B complex act as enzyme cofactors. Thiamin is a cofactor in oxidative decarboxylation of α-keto acids and of transketolase in the pentose phosphate pathway. Riboflavin and niacin are important cofactors in oxidoreduction reactions, present in flavoprotein enzymes and in NAD and NADP, respectively.
![]() Pantothenic acid is present in coenzyme A and acyl carrier protein, which act as carriers for acyl groups in metabolic reactions.
Pantothenic acid is present in coenzyme A and acyl carrier protein, which act as carriers for acyl groups in metabolic reactions.
![]() Vitamin B6 as pyridoxal phosphate is the coenzyme for several enzymes of amino acid metabolism, including the transaminases, and of glycogen phosphorylase. Biotin is the coenzyme for several carboxylase enzymes.
Vitamin B6 as pyridoxal phosphate is the coenzyme for several enzymes of amino acid metabolism, including the transaminases, and of glycogen phosphorylase. Biotin is the coenzyme for several carboxylase enzymes.
![]() Vitamin B12 and folate provide one-carbon residues for DNA synthesis and other reactions; deficiency results in megaloblastic anemia.
Vitamin B12 and folate provide one-carbon residues for DNA synthesis and other reactions; deficiency results in megaloblastic anemia.
![]() Vitamin C is a water-soluble antioxidant that maintains vitamin E and many metal cofactors in the reduced state.
Vitamin C is a water-soluble antioxidant that maintains vitamin E and many metal cofactors in the reduced state.
![]() Inorganic mineral elements that have a function in the body must be provided in the diet. When intake is insufficient, deficiency may develop, and excessive intakes may be toxic.
Inorganic mineral elements that have a function in the body must be provided in the diet. When intake is insufficient, deficiency may develop, and excessive intakes may be toxic.
REFERENCES
Bender DA, Bender AE: Nutrition: A Reference Handbook. Oxford University Press, 1997.
Bender DA: Nutritional Biochemistry of the Vitamins, 2nd ed. Cambridge University Press, 2003.
Department of Health: Dietary Reference Values for Food Energy and Nutrients for the United Kingdom. Her Majesty’s Stationery Office, 1991.
FAO/WHO: Human Vitamin and Mineral Requirements: Report of a Joint FAO/WHO Expert Consultation: Bankok, Thailand. Food and Nutrition Division of the United Nations Food and Agriculture Organization, 2000.
Geissler C, Powers HJ: Human Nutrition, 12th ed. Elsevier, 2010.
Gibney MJ, Lanham-New S, Cassidy A, et al: Introduction to Human Nutrition, The Nutrition Society Textbook Series, 2nd ed. Wiley-Blackwell, 2009.
Institute of Medicine: Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D and Fluoride. National Academy Press, 1997.
Institute of Medicine: Dietary Reference Values for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin and Choline. National Academy Press, 2000.
Institute of Medicine: Dietary Reference Values for Vitamin C, Vitamin E, Selenium and Carotenoids. National Academy Press, 2000.
Institute of Medicine: Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium and Zinc. National Academy Press, 2001.
Scientific Advisory Committee on Nutrition of the Food Standards Agency: Folate and Disease Prevention. The Stationery Office, 2006.