Harper’s Illustrated Biochemistry, 29th Edition (2012)
SECTION VI. Special Topics
Chapter 45. Free Radicals and Antioxidant Nutrients
David A. Bender, PhD
OBJECTIVES
After studying this chapter, you should be able to:
![]() Describe the damage caused to DNA, lipids, and proteins by free radicals, and the diseases associated with radical damage.
Describe the damage caused to DNA, lipids, and proteins by free radicals, and the diseases associated with radical damage.
![]() Describe the main sources of oxygen radicals in the body.
Describe the main sources of oxygen radicals in the body.
![]() Describe the mechanisms and dietary factors that protect against radical damage.
Describe the mechanisms and dietary factors that protect against radical damage.
![]() Explain how antioxidants can also act as pro-oxidants, and why intervention trials of antioxidant nutrients have generally yielded disappointing results.
Explain how antioxidants can also act as pro-oxidants, and why intervention trials of antioxidant nutrients have generally yielded disappointing results.
BIOMEDICAL IMPORTANCE
Free radicals are formed in the body under normal conditions. They cause damage to nucleic acids, proteins, and lipids in cell membranes and plasma lipoproteins. This can cause cancer, atherosclerosis and coronary artery disease, and autoimmune diseases. Epidemiological and laboratory studies have identified a number of protective antioxidant nutrients: selenium, vitamins C and E, β-carotene, and other carotenoids, and a variety of polyphenolic compounds derived from plant foods. Many people take supplements of one or more antioxidant nutrients. However, intervention trials show little benefit of antioxidant supplements except among people who were initially deficient, and many trials of β-carotene and vitamin E have shown increased mortality among those taking the supplements.
Free Radical Reactions Are Self-Perpetuating Chain Reactions
Free radicals are highly reactive molecular species with an unpaired electron; they persist for only a very short time (of the order of 10-9 to 10-12 sec) before they collide with another molecule and either abstract or donate an electron in order to achieve stability. In so doing, they generate a new radical from the molecule with which they collided. The main way in which a free radical can be quenched, so terminating this chain reaction, is if two radicals react together, when the unpaired electrons can become paired in one or other of the parent molecules. This is a rare occurrence, because of the very short half-life of an individual radical and the very low concentrations of radicals in tissues.
The most damaging radicals in biological systems are oxygen radicals (sometimes called reactive oxygen species)—especially superoxide, ![]() , hydroxyl, OH•, and perhydroxyl, O2H• Tissue damage caused by oxygen radicals is often called oxidative damage, and factors that protect against oxygen radical damage are known as antioxidants.
, hydroxyl, OH•, and perhydroxyl, O2H• Tissue damage caused by oxygen radicals is often called oxidative damage, and factors that protect against oxygen radical damage are known as antioxidants.
Radicals Can Damage DNA, Lipids, and Proteins
Interaction of radicals with bases in DNA can lead to chemical changes that, if not repaired (Chapter 35), may be inherited in daughter cells. Radical damage to unsaturated fatty acids in cell membranes and plasma lipoproteins leads to the formation of lipid peroxides, then highly reactive dialdehydes that can chemically modify proteins and nucleic acid bases. Proteins are also subject to direct chemical modification by interaction with radicals. Oxidative damage to tyrosine residues in proteins can lead to the formation of dihydroxyphenylalanine that can undergo nonenzymic reactions leading to further formation of oxygen radicals (Figure 45–1).
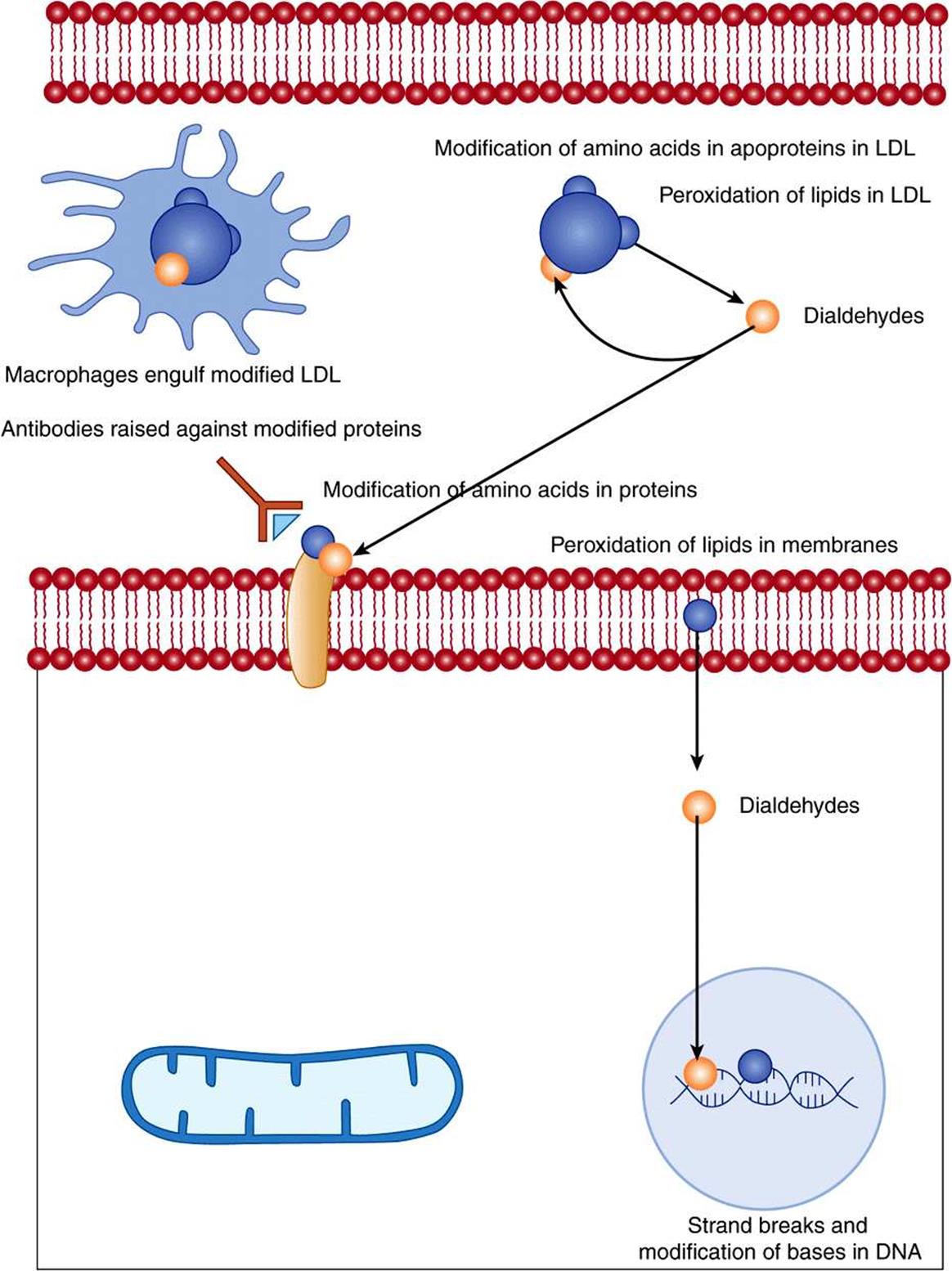
FIGURE 45–1 Tissue damage by radicals.
The total body radical burden can be estimated by measuring the products of lipid peroxidation. Lipid peroxides can be measured by the ferrous oxidation in xylenol orange (FOX) assay. Under acidic conditions, they oxidize Fe2+to Fe3+, which forms a chromophore with xylenol orange. The dialdehydes formed from lipid peroxides can be measured by reaction with thiobarbituric acid, when they form a red fluorescent adduct—the results of this assay are generally reported as total thiobarbituric acid reactive substances, TBARS. Peroxidation of n-6 polyunsaturated fatty acids leads to the formation of pentane, and of n-3 polyunsaturated fatty acids to ethane, both of which can be measured in exhaled air.
Radical Damage May Cause Mutations, Cancer, Autoimmune Disease, and Atherosclerosis
Radical damage to DNA in germline cells in ovaries and testes can lead to heritable mutations; in somatic cells, the result may be initiation of cancer. The dialdehydes formed as a result of radical-induced lipid peroxidation in cell membranes can also modify bases in DNA.
Chemical modification of amino acids in proteins, either by direct radical action or as a result of reaction with the products of radical-induced lipid peroxidation, leads to proteins that are recognized as nonself by the immune system. The resultant antibodies will also cross-react with normal tissue proteins, so initiating autoimmune disease.
Chemical modification of the proteins or lipids in plasma low-density lipoprotein (LDL) leads to abnormal LDL that is not recognized by the liver LDL receptors, and so is not cleared by the liver. The modified LDL is taken up by macrophage scavenger receptors. Lipid-engorged macrophages infiltrate under blood vessel endothelium (especially when there is already some damage to the endothelium), and are killed by the high content of unesterified cholesterol they have accumulated. This occurs in the development of atherosclerotic plaques, which, in extreme cases, can more or less completely occlude a blood vessel.
There Are Multiple Sources of Oxygen Radicals in the Body
Ionizing radiation (x-rays and UV) can lyse water, leading to the formation of hydroxyl radicals. Transition metal ions, including Cu+, Co2+, Ni2+, and Fe2+ can react nonenzymically with oxygen or hydrogen peroxide, again leading to the formation of hydroxyl radicals. Nitric oxide (the endothelium-derived relaxation factor) is itself a radical, and, more importantly, can react with superoxide to yield peroxynitrite, which decays to form hydroxyl radicals (Figure 45–2).
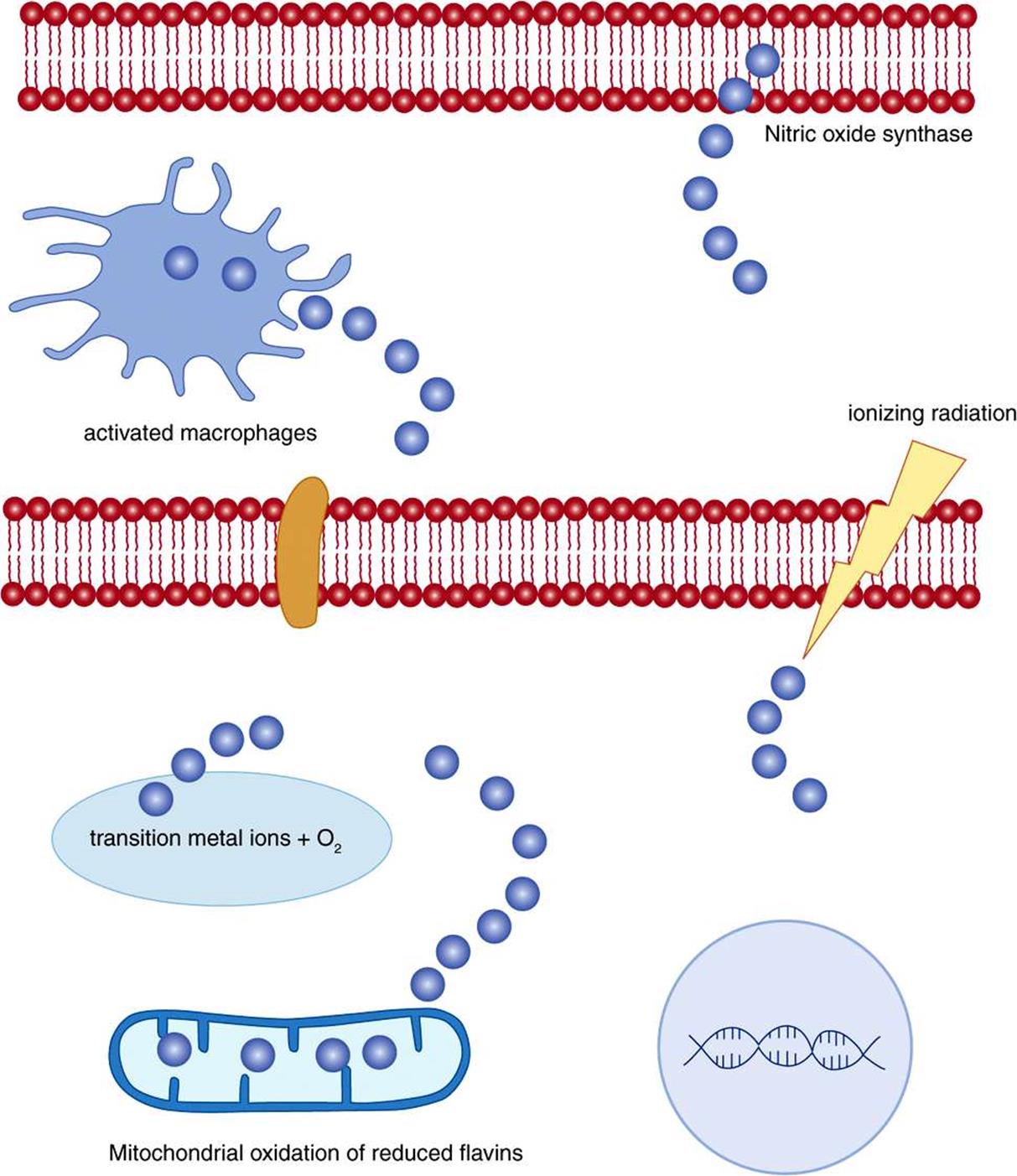
FIGURE 45–2 Sources of radicals.
The respiratory burst of activated macrophages (Chapter 52) is increased utilization of glucose via the pentose phosphate pathway (Chapter 21) to reduce NADP+ to NADPH, and increased utilization of oxygen to oxidise NADPH to produce oxygen (and halogen) radicals as cytotoxic agents to kill phagocytosed microorganisms. The respiratory burst oxidase (NADPH oxidase) is a flavoprotein that reduces oxygen to superoxide: ![]() . Plasma markers of radical damage to lipids increase considerably in response to even a mild infection.
. Plasma markers of radical damage to lipids increase considerably in response to even a mild infection.
The oxidation of reduced flavin coenzymes in the mitochondrial (Chapter 13) and microsomal electron transport chains proceeds through a series of steps in which the flavin semiquinone radical is stabilized by the protein to which it is bound, and forms oxygen radicals as transient intermediates. Although the final products are not radicals, because of the unpredictable nature of radicals there is considerable “leakage” of radicals, and some 3%-5% of the daily consumption of 30 mol of oxygen by an adult human being is converted to singlet oxygen, hydrogen peroxide, and superoxide, perhydroxyl, and hydroxyl radicals, rather then undergoing complete reduction to water. This results in daily production of about 1.5 mol of reactive oxygen species.
There Are Various Mechanisms of Protection Against Radical Damage
The metal ions that undergo nonenzymic reaction to form oxygen radicals are not normally free in solution, but are bound to either the proteins for which they provide the prosthetic group, or to specific transport and storage proteins, so that they are unreactive. Iron is bound to transferrin, ferritin, and hemosiderin, copper to ceruloplasmin, and other metal ions are bound to metallothionein. This binding to transport proteins that are too large to be filtered in the kidneys also prevents loss of metal ions in the urine.
Superoxide is produced both accidentally and also as the reactive oxygen species required for a number of enzyme-catalyzed reactions. A family of superoxide dismutases catalyze the reaction between superoxide and protons to yield oxygen and hydrogen peroxide: ![]() . The hydrogen peroxide is then removed by catalase and various peroxidases: 2H2O2 → 2H2O + O2. Most enzymes that produce and require superoxide are in the peroxisomes, together with superoxide dismutase, catalase, and peroxidases.
. The hydrogen peroxide is then removed by catalase and various peroxidases: 2H2O2 → 2H2O + O2. Most enzymes that produce and require superoxide are in the peroxisomes, together with superoxide dismutase, catalase, and peroxidases.
The peroxides that are formed by radical damage to lipids in membranes and plasma lipoproteins are reduced to hydroxy fatty acids by glutathione peroxidase, a selenium-dependent enzyme (hence the importance of adequate selenium intake to maximize antioxidant activity), and the oxidized glutathione is reduced by NADPH-dependent glutathione reductase (Figure 21–3). Lipid peroxides are also reduced to fatty acids by reaction with vitamin E, forming the relatively stable tocopheroxyl radical, which persist long enough to undergo reduction back to tocopherol by reaction with vitamin C at the surface of the cell or lipoprotein (Figure 44–6). The resultant monodehydroascorbate radical then undergoes enzymic reduction back to ascorbate or a nonenzymic reaction of 2 mol of monodehydroascorbate to yield 1 mol each of ascorbate and dehydroascorbate.
Ascorbate, uric acid and a variety of polyphenols derived from plant foods act as water-soluble radical trapping antioxidants, forming relatively stable radicals that persist long enough to undergo reaction to nonradical products. Ubiquinone and carotenes similarly act as lipid-soluble radical-trapping antioxidants in membranes and plasma lipoproteins.
Antioxidants Can Also Be Pro-Oxidants
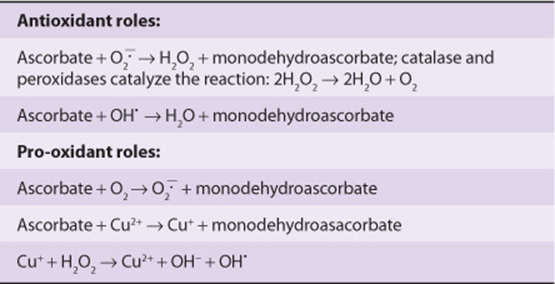
Although ascorbate is an antioxidant, reacting with superoxide and hydroxyl to yield monodehydroascorbate and hydrogen peroxide or water, it can also be a source of superoxide radicals by reaction with oxygen, and hydroxyl radicals by reaction with Cu2+ ions (Table 45-1). However, these pro-oxidant actions require relatively high concentrations of ascorbate that are unlikely to be reached in tissues, since once the plasma concentration of ascorbate reaches about 30 mmol/L, the renal threshold is reached, and at intakes above about 100-120 mg/day the vitamin is excreted in the urine quantitatively with intake.
TABLE 45–1 Antioxidant and Pro-Oxidant Roles of Vitamin C
A considerable body of epidemiological evidence suggested that carotene is protective against lung and other cancers. However, two major intervention trials in the 1990s showed an increase in death from lung (and other) cancer among people given supplements of β-carotene. The problem is that although β-carotene is indeed a radical-trapping antioxidant under conditions of low partial pressure of oxygen, as in most tissues, at high partial pressures of oxygen (as in the lungs) and especially in high concentrations, β-carotene is an autocatalytic pro-oxidant, and hence can initiate radical damage to lipids and proteins.
Epidemiological evidence also suggests that vitamin E is protective against atherosclerosis and cardiovascular disease. However, meta-analysis of intervention trials with vitamin E shows increased mortality among those taking (high dose) supplements. These trials have all used α-tocopherol, and it is possible that the other vitamers of vitamin E that are present in foods, but not the supplements, may be important. In vitro, plasma lipoproteins form less cholesterol ester hydroperoxide when incubated with sources of low concentrations of perhydroxyl radicals when vitamin E has been removed than when it is present. The problem seems to be that vitamin E acts as an antioxidant by forming a stable radical that persists long enough to undergo metabolism to nonradical products. This means that the radical also persists long enough to penetrate deeper in to the lipoprotein, causing further radical damage, rather than interacting with a water-soluble antioxidant at the surface of the lipoprotein.
SUMMARY
![]() Free radicals are highly reactive molecular species with an unpaired electron. They can react with, and modify, proteins, nucleic acids and fatty acids in cell membranes and plasma lipoproteins.
Free radicals are highly reactive molecular species with an unpaired electron. They can react with, and modify, proteins, nucleic acids and fatty acids in cell membranes and plasma lipoproteins.
![]() Radical damage to lipids and proteins in plasma lipoproteins is a factor in the development of atherosclerosis and coronary artery disease; radical damage to nucleic acids may induce heritable mutations and cancer; radical damage to proteins may lead to the development of autoimmune diseases.
Radical damage to lipids and proteins in plasma lipoproteins is a factor in the development of atherosclerosis and coronary artery disease; radical damage to nucleic acids may induce heritable mutations and cancer; radical damage to proteins may lead to the development of autoimmune diseases.
![]() Oxygen radicals arise as a result of exposure to ionizing radiation, nonenzymic reactions of transition metal ions, the respiratory burst of activated macrophages, and the normal oxidation of reduced flavin coenzymes.
Oxygen radicals arise as a result of exposure to ionizing radiation, nonenzymic reactions of transition metal ions, the respiratory burst of activated macrophages, and the normal oxidation of reduced flavin coenzymes.
![]() Protection against radical damage is afforded by enzymes that remove superoxide ions and hydrogen peroxide, enzymic reduction of lipid peroxides linked to oxidation of glutathione, nonenzymic reaction of lipid peroxides with vitamin E, and reaction of radicals with compounds such as vitamins C and E, carotene, ubiquinone, uric acid, and dietary polyphenols that form relatively stable radicals that persist long enough to undergo reaction to nonradical products.
Protection against radical damage is afforded by enzymes that remove superoxide ions and hydrogen peroxide, enzymic reduction of lipid peroxides linked to oxidation of glutathione, nonenzymic reaction of lipid peroxides with vitamin E, and reaction of radicals with compounds such as vitamins C and E, carotene, ubiquinone, uric acid, and dietary polyphenols that form relatively stable radicals that persist long enough to undergo reaction to nonradical products.
![]() Except in people who were initially deficient, intervention trials of vitamin E and β-carotene have generally shown increased mortality among those taking the supplements. β-Carotene is only an antioxidant at low concentrations of oxygen; at higher concentrations of oxygen it is an autocatalytic prooxidant. Vitamin E forms a stable radical that is capable of either undergoing reaction with water-soluble antioxidants or penetrating further into lipoproteins and tissues, so increasing radical damage.
Except in people who were initially deficient, intervention trials of vitamin E and β-carotene have generally shown increased mortality among those taking the supplements. β-Carotene is only an antioxidant at low concentrations of oxygen; at higher concentrations of oxygen it is an autocatalytic prooxidant. Vitamin E forms a stable radical that is capable of either undergoing reaction with water-soluble antioxidants or penetrating further into lipoproteins and tissues, so increasing radical damage.
REFERENCES
Asplund K: Antioxidant vitamins in the prevention of cardiovascular disease: a systematic review. J Intern Med 2002;251:372.
Bjelakovic G, Nikolova D, Gluud LL, et al: Mortality in randomised trials of antioxidant supplements for primary and secondary prevention. JAMA 2007;297:842.
Burton G, Ingold K: β-Carotene, an unusual type of lipid antioxidant. Science 1984;224:569.
Carr A, Frei B: Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J 1999;13:1007.
Cordero Z, Drogan D, Weikert C, et al: Vitamin E and risk of cardiovascular diseases: a review of epidemiologic and clinical trial studies. Crit Rev Food Sci Nutr 2010;50;420.
Dotan Y, Lichtenberg D, Pinchuk I: No evidence supports vitamin E indiscriminate supplementation. Biofactors 2009;35:469.
Halliwell B, Gutteridge JM, Cross CE: Free radicals, antioxidants and human disease: where are we now? J Lab Clin Med 1992;119:598.
Imlay JA: Pathways of oxidative damage. Ann Rev Microbiol 2003;57:395.
Imlay JA: Cellular Defenses against superoxide and hydrogen peroxide. Ann Rev Biochem 2008;77:755.
Klaunig JE, Kamendulis LM: The role of oxidative stress in carcinogenesis. Ann Rev Pharm Tox 2004;44:239.
Miller ER, Pastor-Barriuso R, Dalal D, et al: Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 2005;142:37.
Omenn GS, Goodman GE, Thornquist MD, et al: Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med 1996;334:1150.
Various authors: Symposium: Antioxidant vitamins and β-carotene in disease prevention. Amer J Clin Nutr 1995;62(suppl 6): 12995-15405.
Various authors: Symposium Proceedings: Molecular mechanisms of protective effects of vitamin E in atherosclerosis. J Nutr 2001;131:366-397.