CHEMISTRY THE CENTRAL SCIENCE
11 LIQUIDS AND INTERMOLECULAR FORCES
11.2 INTERMOLECULAR FORCES
The strengths of intermolecular forces in different substances vary over a wide range but are generally much weaker than intramolecular forces—ionic, metallic or covalent bonds (![]() FIGURE 11.3). Less energy, therefore, is required to vaporize a liquid or melt a solid than to break covalent bonds. For example, only 16 kJ/mol is required to overcome the intermolecular attractions in liquid HCl in order to vaporize it. In contrast, the energy required to break the covalent bond in HCl is 431 kJ/mol. Thus, when a molecular substance such as HCl changes from solid to liquid to gas, the molecules remain intact.
FIGURE 11.3). Less energy, therefore, is required to vaporize a liquid or melt a solid than to break covalent bonds. For example, only 16 kJ/mol is required to overcome the intermolecular attractions in liquid HCl in order to vaporize it. In contrast, the energy required to break the covalent bond in HCl is 431 kJ/mol. Thus, when a molecular substance such as HCl changes from solid to liquid to gas, the molecules remain intact.
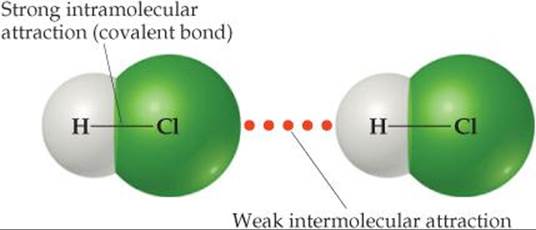
![]() FIGURE 11.3 Intermolecular and intramolecular forces
FIGURE 11.3 Intermolecular and intramolecular forces
Many properties of liquids, including boiling points, reflect the strength of the intermolecular forces. A liquid boils when bubbles of its vapor form within the liquid. The molecules of the liquid must overcome their attractive forces in order to separate and form a vapor. The stronger the attractive forces, the higher the temperature at which the liquid boils. Similarly, the melting points of solids increase as the strengths of the intermolecular forces increase. As shown in ![]() TABLE 11.2, the melting and boiling points of substances in which the particles are held together by chemical bonds tend to be much higher than those of substances in which the particles are held together by intermolecular forces.
TABLE 11.2, the melting and boiling points of substances in which the particles are held together by chemical bonds tend to be much higher than those of substances in which the particles are held together by intermolecular forces.
Three types of intermolecular attractions exist between electrically neutral molecules: dispersion forces, dipole–dipole attractions, and hydrogen bonding. The first two are collectively called van der Waals forces after Johannes van der Waals, who developed the equation for predicting the deviation of gases from ideal behavior. ![]() (Section 10.9) Another kind of attractive force, the ion-dipole force, is important in solutions.
(Section 10.9) Another kind of attractive force, the ion-dipole force, is important in solutions.
All intermolecular interactions are electrostatic, involving attractions between positive and negative species, much like ionic bonds. ![]() (Section 8.2) Why then are intermolecular forces so much weaker than ionic bonds? Recall from Equation 8.4 that electrostatic interactions get stronger as the magnitude of the charges increases and weaker as the distance between charges increases. The charges responsible for intermolecular forces are generally much smaller than the charges in ionic compounds. For example, from its dipole moment it is possible to estimate charges of +0.178 and –0.178 for the hydrogen and chlorine ends of the HCl molecule (see Sample Exercise 8.5). Furthermore, the distances between molecules are often larger than the distances between atoms held together by chemical bonds.
(Section 8.2) Why then are intermolecular forces so much weaker than ionic bonds? Recall from Equation 8.4 that electrostatic interactions get stronger as the magnitude of the charges increases and weaker as the distance between charges increases. The charges responsible for intermolecular forces are generally much smaller than the charges in ionic compounds. For example, from its dipole moment it is possible to estimate charges of +0.178 and –0.178 for the hydrogen and chlorine ends of the HCl molecule (see Sample Exercise 8.5). Furthermore, the distances between molecules are often larger than the distances between atoms held together by chemical bonds.
TABLE 11.2 • Melting and Boiling Points of Representative Substances
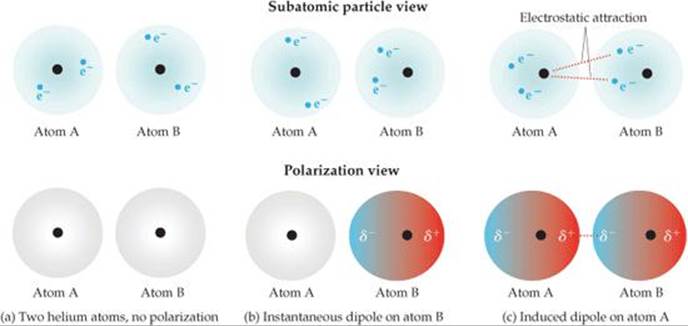
![]() FIGURE 11.4 Dispersion forces. “Snapshots” of the charge distribution for a pair of helium atoms at three instants.
FIGURE 11.4 Dispersion forces. “Snapshots” of the charge distribution for a pair of helium atoms at three instants.
Dispersion Forces
You might think there would be no electrostatic interactions between electrically neutral, nonpolar atoms and/or molecules. Yet some kind of attractive interactions must exist because nonpolar gases like helium, argon, and nitrogen can be liquefied. Fritz London, a German-American physicist, first proposed the origin of this attraction in 1930. London recognized that the motion of electrons in an atom or molecule can create an instantaneous, or momentary, dipole moment.
In a collection of helium atoms, for example, the average distribution of the electrons about each nucleus is spherically symmetrical, as shown in ![]() FIGURE 11.4 (a). The atoms are nonpolar and so possess no permanent dipole moment. The instantaneous distribution of the electrons, however, can be different from the average distribution. If we could freeze the motion of the electrons at any given instant, both electrons could be on one side of the nucleus. At just that instant, the atom has an instantaneous dipole moment as shown in Figure 11.4(b). The motions of electrons in one atom influence the motions of electrons in its neighbors. The instantaneous dipole on one atom can induce an instantaneous dipole on an adjacent atom, causing the atoms to be attracted to each other as shown in Figure 11.4(c). This attractive interaction is called thedispersion force (or the London dispersion force in some texts). It is significant only when molecules are very close together.
FIGURE 11.4 (a). The atoms are nonpolar and so possess no permanent dipole moment. The instantaneous distribution of the electrons, however, can be different from the average distribution. If we could freeze the motion of the electrons at any given instant, both electrons could be on one side of the nucleus. At just that instant, the atom has an instantaneous dipole moment as shown in Figure 11.4(b). The motions of electrons in one atom influence the motions of electrons in its neighbors. The instantaneous dipole on one atom can induce an instantaneous dipole on an adjacent atom, causing the atoms to be attracted to each other as shown in Figure 11.4(c). This attractive interaction is called thedispersion force (or the London dispersion force in some texts). It is significant only when molecules are very close together.
The strength of the dispersion force depends on the ease with which the charge distribution in a molecule can be distorted to induce an instantaneous dipole. The ease with which the charge distribution is distorted is called the molecule's polarizability. We can think of the polarizability of a molecule as a measure of the “squashiness” of its electron cloud: The greater the polarizability, the more easily the electron cloud can be distorted to give an instantaneous dipole. Therefore, more polarizable molecules have larger dispersion forces.
In general, polarizability increases as the number of electrons in an atom or molecule increases. The strength of dispersion forces therefore tends to increase with increasing atomic or molecular size. Because molecular size and mass generally parallel each other, dispersion forces tend to increase in strength with increasing molecular weight. We can see this in the boiling points of the halogens and noble gases (![]() FIGURE 11.5), where dispersion forces are the only intermolecular forces at work. In both families the molecular weight increases on moving down the periodic table. The higher molecular weights translate into stronger dispersion forces, which in turn lead to higher boiling points.
FIGURE 11.5), where dispersion forces are the only intermolecular forces at work. In both families the molecular weight increases on moving down the periodic table. The higher molecular weights translate into stronger dispersion forces, which in turn lead to higher boiling points.
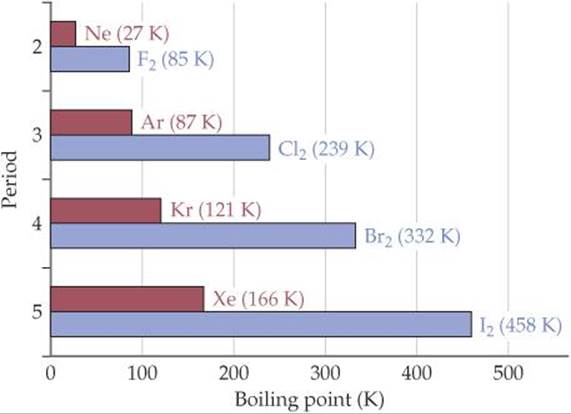
![]() FIGURE 11.5 Boiling points of the halogens and noble gases. This plot shows how the boiling points increase as the molecular weight increases due to stronger dispersion forces.
FIGURE 11.5 Boiling points of the halogens and noble gases. This plot shows how the boiling points increase as the molecular weight increases due to stronger dispersion forces.
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
List the substances CCl4, CBr4, and CH4 in order of increasing boiling point.
Molecular shape also influences the magnitudes of dispersion forces. For example, n-pentane* and neopentane (![]() FIGURE 11.6) have the same molecular formula (C5H12), yet the boiling point of n-pentane is about 27 K higher than that of neopentane. The difference can be traced to the different shapes of the two molecules. Intermolecular attraction is greater for n-pentane because the molecules can come in contact over the entire length of the long, somewhat cylindrical molecules. Less contact is possible between the more compact and nearly spherical neopentane molecules.
FIGURE 11.6) have the same molecular formula (C5H12), yet the boiling point of n-pentane is about 27 K higher than that of neopentane. The difference can be traced to the different shapes of the two molecules. Intermolecular attraction is greater for n-pentane because the molecules can come in contact over the entire length of the long, somewhat cylindrical molecules. Less contact is possible between the more compact and nearly spherical neopentane molecules.
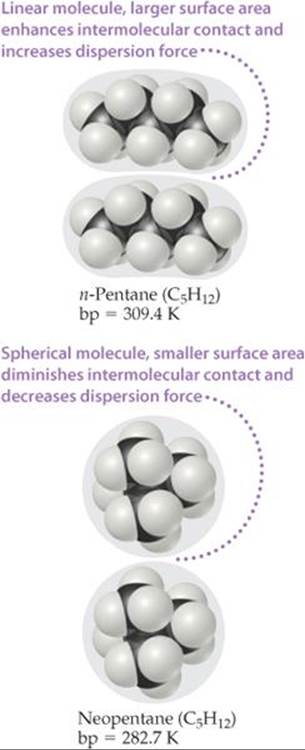
![]() FIGURE 11.6 Molecular shape affects intermolecular attraction. Molecules of n-pentane make more contact with each other than do neopentane molecules. Thus, n-pentane has stronger intermolecular attractive forces and a higher boiling point.
FIGURE 11.6 Molecular shape affects intermolecular attraction. Molecules of n-pentane make more contact with each other than do neopentane molecules. Thus, n-pentane has stronger intermolecular attractive forces and a higher boiling point.
Dipole–Dipole Forces
The presence of a permanent dipole moment in polar molecules gives rise to dipole-dipole forces. These forces originate from electrostatic attractions between the partially positive end of one molecule and partially negative end of a neighboring molecule. Repulsions can also occur when the positive (or negative) ends of two molecules are in close proximity. Dipole-dipole forces are effective only when molecules are very close together.
To see the effect of dipole-dipole forces, we compare the boiling points of two compounds of similar molecular weight: acetonitrile (CH3CN, MW 41 amu, bp 355 K) and propane (CH3CH2CH3, MW 44 amu, bp 231 K). Acetonitrile is a polar molecule, with a dipole moment of 3.9 D, so dipole-dipole forces are present. However, propane is essentially nonpolar, which means that dipole-dipole forces are absent. Because acetonitrile and propane have similar molecular weights, dispersion forces are similar for these two molecules. Therefore, the higher boiling point of acetonitrile can be attributed to dipole-dipole forces.
To better understand these forces, consider how CH3CN molecules pack together in the solid and liquid states. In the solid [![]() FIGURE 11.7 (a)], the molecules are arranged with the negatively charged nitrogen end of each molecule close to the positively charged —CH3 ends of its neighbors. In the liquid [Figure 11.7(b)], the molecules are free to move with respect to one another, and their arrangement becomes more disordered. This means that, at any given instant, both attractive and repulsive dipole-dipole interactions are present. However, not only are there more attractive interactions than repulsive ones, but also molecules that are attracting each other spend more time near each other than do molecules that are repelling each other. The overall effect is a net attraction strong enough to keep the molecules in liquid CH3CN from moving apart to form a gas.
FIGURE 11.7 (a)], the molecules are arranged with the negatively charged nitrogen end of each molecule close to the positively charged —CH3 ends of its neighbors. In the liquid [Figure 11.7(b)], the molecules are free to move with respect to one another, and their arrangement becomes more disordered. This means that, at any given instant, both attractive and repulsive dipole-dipole interactions are present. However, not only are there more attractive interactions than repulsive ones, but also molecules that are attracting each other spend more time near each other than do molecules that are repelling each other. The overall effect is a net attraction strong enough to keep the molecules in liquid CH3CN from moving apart to form a gas.
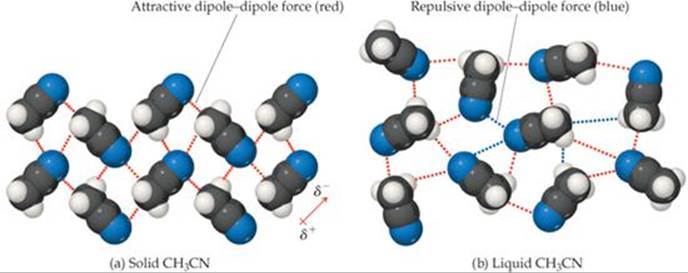
![]() FIGURE 11.7 Dipole-dipole interactions. The dipole-dipole interactions in (a) crystalline CH3CN and (b) liquid CH3CN.
FIGURE 11.7 Dipole-dipole interactions. The dipole-dipole interactions in (a) crystalline CH3CN and (b) liquid CH3CN.
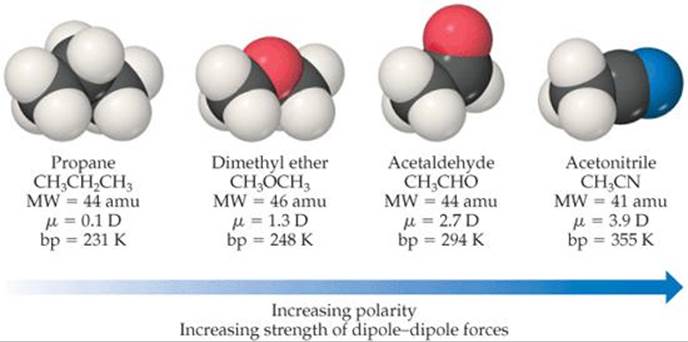
![]() FIGURE 11.8 Molecular weights, dipole moments, and boiling points of several simple organic substances.
FIGURE 11.8 Molecular weights, dipole moments, and boiling points of several simple organic substances.
For molecules of approximately equal mass and size, the strength of intermolecular attractions increases with increasing polarity, a trend we see in ![]() FIGURE 11.8. Notice how the boiling point increases as the dipole moment increases.
FIGURE 11.8. Notice how the boiling point increases as the dipole moment increases.
Hydrogen Bonding
![]() FIGURE 11.9 shows the boiling points of the binary compounds that form between hydrogen and the elements in groups 4A through 7A. The boiling points of the compounds containing group 4A elements (CH4 through SnH4, all nonpolar) increase systematically moving down the group. This is the expected trend because polarizability and, hence, dispersion forces generally increase as molecular weight increases. The three heavier members of groups 5A, 6A, and 7A follow the same trend, but NH3, H2O, and HF have boiling points that are much higher than expected. In fact, these three compounds also have many other characteristics that distinguish them from other substances of similar molecular weight and polarity. For example, water has a high melting point, a high specific heat, and a high heat of vaporization. Each of these properties indicates that the intermolecular forces are abnormally strong.
FIGURE 11.9 shows the boiling points of the binary compounds that form between hydrogen and the elements in groups 4A through 7A. The boiling points of the compounds containing group 4A elements (CH4 through SnH4, all nonpolar) increase systematically moving down the group. This is the expected trend because polarizability and, hence, dispersion forces generally increase as molecular weight increases. The three heavier members of groups 5A, 6A, and 7A follow the same trend, but NH3, H2O, and HF have boiling points that are much higher than expected. In fact, these three compounds also have many other characteristics that distinguish them from other substances of similar molecular weight and polarity. For example, water has a high melting point, a high specific heat, and a high heat of vaporization. Each of these properties indicates that the intermolecular forces are abnormally strong.
![]() GO FIGURE
GO FIGURE
Why is the boiling point of SnH4 higher than that of CH4?
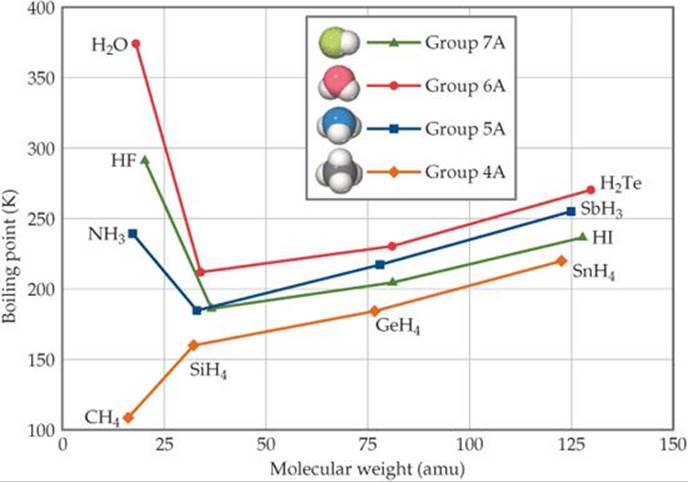
![]() FIGURE 11.9 Boiling points of the covalent hydrides of the elements in groups 4A–7A as a function of molecular weight.
FIGURE 11.9 Boiling points of the covalent hydrides of the elements in groups 4A–7A as a function of molecular weight.
![]() GO FIGURE
GO FIGURE
To form a hydrogen bond what must the non-hydrogen atom (N, O, or F) involved in the bond possess?
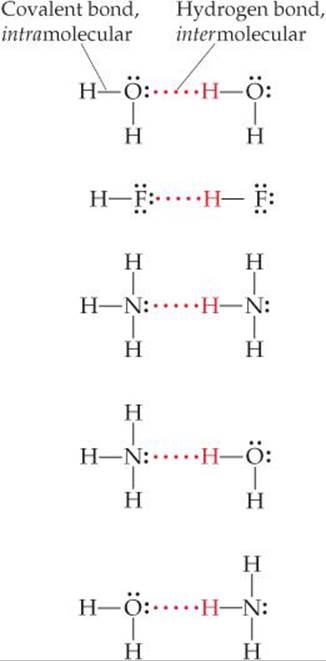
![]() FIGURE 11.10 Hydrogen bonding.
FIGURE 11.10 Hydrogen bonding.
The strong intermolecular attractions in HF, H2O, and NH3 result from hydrogen bonding. Hydrogen bonding is a special type of intermolecular attraction between the hydrogen atom in a polar bond (particularly H—F, H—O, and H—N) and nonbonding electron pair on a nearby small electronegative ion or atom usually F, O, or N (in another molecule). For example, a hydrogen bond exists between the H atom in an HF molecule and the F atom of an adjacent HF molecule, as shown in ![]() FIGURE 11.10 along with several additional examples.
FIGURE 11.10 along with several additional examples.
Hydrogen bonds can be considered a type of dipole-dipole attraction. Because N, O, and F are so electronegative, a bond between hydrogen and any of these elements is quite polar, with hydrogen at the positive end (remember the + on the right-hand side of the dipole symbol represents the positive end of the dipole):
![]()
The hydrogen atom has no inner electrons. Thus, the positive side of the dipole has the concentrated charge of the nearly bare hydrogen nucleus. This positive charge is attracted to the negative charge of an electronegative atom in a nearby molecule. Because the electron-poor hydrogen is so small, it can approach an electronegative atom very closely and, thus, interact strongly with it.
SAMPLE EXERCISE 11.1 Identifying Substances That Can Form Hydrogen Bonds
In which of these substances is hydrogen bonding likely to play an important role in determining physical properties: methane (CH4), hydrazine (H2NNH2), methyl fluoride (CH3F), hydrogen sulfide (H2S)?
SOLUTION
Analyze We are given the chemical formulas of four compounds and asked to predict whether they can participate in hydrogen bonding. All the compounds contain H, but hydrogen bonding usually occurs only when the hydrogen is covalently bonded to N, O, or F.
Plan We analyze each formula to see if it contains N, O, or F directly bonded to H. There also needs to be a nonbonding pair of electrons on an electronegative atom (usually N, O, or F) in a nearby molecule, which can be revealed by drawing the Lewis structure for the molecule.
Solve The foregoing criteria eliminate CH4 and H2S, which do not contain H bonded to N, O, or F. They also eliminate CH3F, whose Lewis structure shows a central C atom surrounded by three H atoms and an F atom. (Carbon always forms four bonds, whereas hydrogen and fluorine form one each.) Because the molecule contains a C—F bond and not a H—F bond, it does not form hydrogen bonds. In H2NNH2, however, we find N—H bonds, and the Lewis structure shows a nonbonding pair of electrons on each N atom, telling us hydrogen bonds can exist between the molecules:
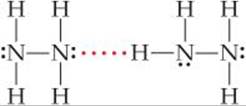
Check Although we can generally identify substances that participate in hydrogen bonding based on their containing N, O, or F covalently bonded to H, drawing the Lewis structure for the interaction provides a way to check the prediction.
PRACTICE EXERCISE
In which of these substances is significant hydrogen bonding possible: methylene chloride (CH2Cl2), phosphine (PH3), hydrogen peroxide (HOOH), acetone (CH3COCH3)?
Answer: HOOH

Calvin and Hobbes © Watterson, Dist. by Universal Press Syndicate. Reprinted with permission. All rights reserved.
The energies of hydrogen bonds vary from about 5 kJ/mol to 25 kJ/mol, although there are isolated examples of hydrogen bond energies close to 100 kJ/mol. Thus, hydrogen bonds are typically much weaker than covalent bonds, which have bond enthalpies of 150–1100 kJ/mol (seeTable 8.4). Nevertheless, because hydrogen bonds are generally stronger than dipole-dipole or dispersion forces, they play important roles in many chemical systems, including those of biological significance. For example, hydrogen bonds help stabilize the structures of proteins and are also responsible for the way that DNA is able to carry genetic information.
One remarkable consequence of hydrogen bonding is seen in the densities of ice and liquid water. In most substances the molecules in the solid are more densely packed than in the liquid, making the solid phase denser than the liquid phase. By contrast, the density of ice at 0 °C (0.917 g/mL) is less than that of liquid water at 0 °C (1.00 g/mL), so ice floats on liquid water.
The lower density of ice can be understood in terms of hydrogen bonding. In ice, the H2O molecules assume the ordered, open arrangement shown in ![]() FIGURE 11.11. This arrangement optimizes hydrogen bonding between molecules, with each H2O molecule forming hydrogen bonds to four neighboring H2O molecules. These hydrogen bonds, however, create the cavities seen in the middle image of Figure 11.11. When ice melts, the motions of the molecules cause the structure to collapse. The hydrogen bonding in the liquid is more random than in the solid but is strong enough to hold the molecules close together. Consequently, liquid water has a denser structure than ice, meaning that a given mass of water occupies a smaller volume than the same mass of ice.
FIGURE 11.11. This arrangement optimizes hydrogen bonding between molecules, with each H2O molecule forming hydrogen bonds to four neighboring H2O molecules. These hydrogen bonds, however, create the cavities seen in the middle image of Figure 11.11. When ice melts, the motions of the molecules cause the structure to collapse. The hydrogen bonding in the liquid is more random than in the solid but is strong enough to hold the molecules close together. Consequently, liquid water has a denser structure than ice, meaning that a given mass of water occupies a smaller volume than the same mass of ice.
![]() GO FIGURE
GO FIGURE
What is the approximate H—O····H bond angle in ice, where H—O is the covalent bond and O····H is the hydrogen bond?
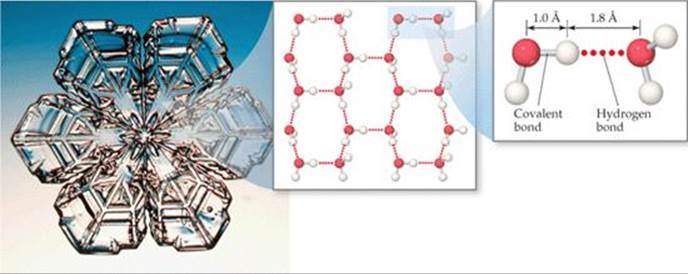
![]() FIGURE 11.11 Hydrogen bonding in ice. The empty channels in the structure of ice make water less dense as a solid than as a liquid.
FIGURE 11.11 Hydrogen bonding in ice. The empty channels in the structure of ice make water less dense as a solid than as a liquid.
The expansion of water upon freezing (![]() FIGURE 11.12) is responsible for many phenomena we take for granted. It causes icebergs to float and water pipes to burst in cold weather. The lower density of ice compared to liquid water also profoundly affects life on Earth. Because ice floats, it covers the top of the water when a lake freezes, thereby insulating the water. If ice were denser than water, ice forming at the top of a lake would sink to the bottom, and the lake could freeze solid. Most aquatic life could not survive under these conditions.
FIGURE 11.12) is responsible for many phenomena we take for granted. It causes icebergs to float and water pipes to burst in cold weather. The lower density of ice compared to liquid water also profoundly affects life on Earth. Because ice floats, it covers the top of the water when a lake freezes, thereby insulating the water. If ice were denser than water, ice forming at the top of a lake would sink to the bottom, and the lake could freeze solid. Most aquatic life could not survive under these conditions.

![]() FIGURE 11.12 Expansion of water upon freezing.
FIGURE 11.12 Expansion of water upon freezing.
Ion–Dipole Forces
An ion–dipole force exists between an ion and a polar molecule (![]() FIGURE 11.13). Cations are attracted to the negative end of a dipole, and anions are attracted to the positive end. The magnitude of the attraction increases as either the ionic charge or the magnitude of the dipole moment increases. Ion-dipole forces are especially important for solutions of ionic substances in polar liquids, such as a solution of NaCl in water.
FIGURE 11.13). Cations are attracted to the negative end of a dipole, and anions are attracted to the positive end. The magnitude of the attraction increases as either the ionic charge or the magnitude of the dipole moment increases. Ion-dipole forces are especially important for solutions of ionic substances in polar liquids, such as a solution of NaCl in water. ![]() (Section 4.1)
(Section 4.1)
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
In which mixture do you expect to find ion-dipole forces: CH3OH in water or Ca(NO3)2 in water?
Comparing Intermolecular Forces
We can identify the intermolecular forces operative in a substance by considering its composition and structure. Dispersion forces are found in all substances. The strength of these attractive forces increases with increasing molecular weight and depends on molecular shapes. With polar molecules dipole–dipole forces are also operative, but these forces often make a smaller contribution to the total intermolecular attraction than dispersion forces. For example, in liquid HCl dispersion forces are estimated to account for more than 80% of the total attraction between molecules, while dipole–dipole attractions account for the rest. Hydrogen bonds, when present, make an important contribution to the total intermolecular interaction. In general, the energies associated with dispersion and dipole-dipole forces are 2–10 kJ/mol, while the energies of hydrogen bonds are 5–25 kJ/mol. Ion–dipole attractions have energies of approximately 15 kJ/mol. All these interactions are considerably weaker than covalent and ionic bonds, which have energies that are hundreds of kilojoules per mole.
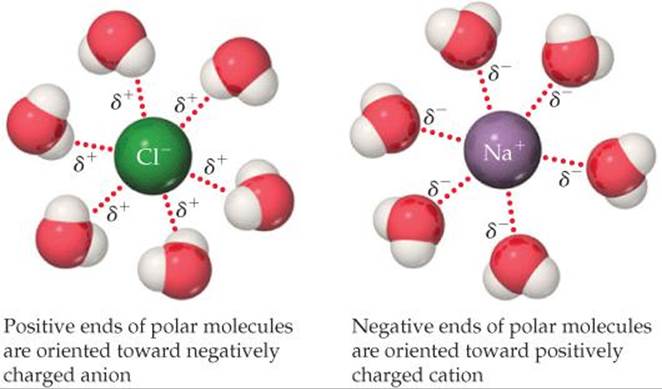
![]() FIGURE 11.13 Ion-dipole forces.
FIGURE 11.13 Ion-dipole forces.
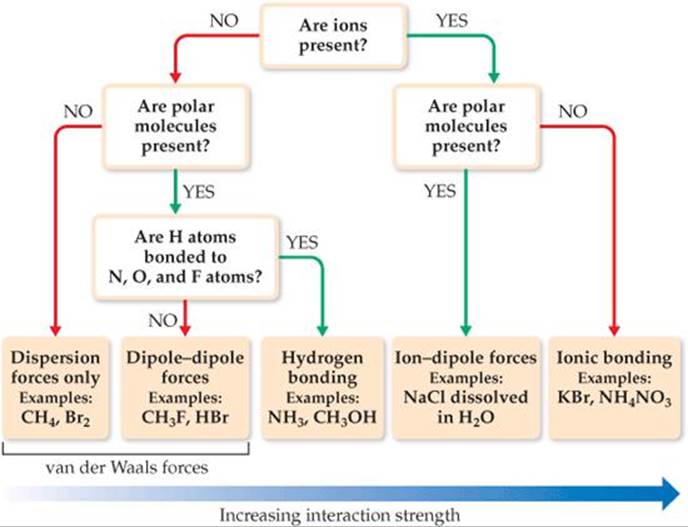
![]() FIGURE 11.14 Flowchart for determining intermolecular forces. Multiple types of intermolecular forces can be at work in a given substance or mixture. In particular, dispersion forces occur in all substances.
FIGURE 11.14 Flowchart for determining intermolecular forces. Multiple types of intermolecular forces can be at work in a given substance or mixture. In particular, dispersion forces occur in all substances.
When comparing the relative strengths of intermolecular attractions, consider these generalizations:
1. When the molecules of two substances have comparable molecular weights and shapes, dispersion forces are approximately equal in the two substances. Differences in the magnitudes of the intermolecular forces are due to differences in the strengths of dipole–dipole attractions. The intermolecular forces get stronger as molecule polarity increases, with those molecules capable of hydrogen bonding having the strongest interactions.
2. When the molecules of two substances differ widely in molecular weights, dispersion forces tend to determine which substance has the stronger intermolecular attractions. Intermolecular attractive forces are generally higher in the substance with higher molecular weight.
![]() FIGURE 11.14 presents a systematic way of identifying the intermolecular forces in a particular system.
FIGURE 11.14 presents a systematic way of identifying the intermolecular forces in a particular system.
It is important to realize that the effects of all these attractions are additive. For example, acetic acid, CH3COOH, and 1-propanol, CH3CH2CH2OH, have the same molecular weight, 60 amu, and both are capable of forming hydrogen bonds. However, a pair of acetic acid molecules can form two hydrogen bonds, whereas a pair of 1-propanol molecules can form only one (![]() FIGURE 11.15). Hence, the boiling point of acetic acid is higher. These effects can be important, especially for very large polar molecules such as proteins, which have multiple dipoles over their surfaces. These molecules can be held together in solution to a surprisingly high degree due to the presence of multiple dipole–dipole attractions.
FIGURE 11.15). Hence, the boiling point of acetic acid is higher. These effects can be important, especially for very large polar molecules such as proteins, which have multiple dipoles over their surfaces. These molecules can be held together in solution to a surprisingly high degree due to the presence of multiple dipole–dipole attractions.
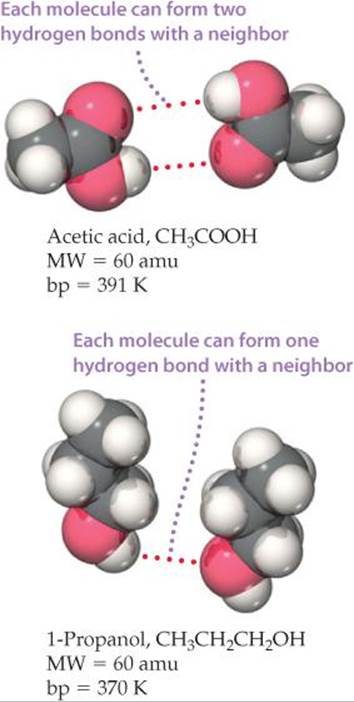
![]() FIGURE 11.15 Hydrogen bonding in acetic acid and 1-propanol. The greater the number of hydrogen bonds possible, the more tightly the molecules are held together and, therefore, the higher the boiling point.
FIGURE 11.15 Hydrogen bonding in acetic acid and 1-propanol. The greater the number of hydrogen bonds possible, the more tightly the molecules are held together and, therefore, the higher the boiling point.
SAMPLE EXERCISE 11.2 Predicting Types and Relative Strengths of Intermolecular Attractions
List the substances BaCl2, H2, CO, HF, and Ne in order of increasing boiling point.
SOLUTION
Analyze We need to assess the intermolecular forces in these substances and use that information to determine the relative boiling points.
Plan The boiling point depends in part on the attractive forces in each substance. We need to order these according to the relative strengths of the different kinds of intermolecular attractions.
Solve The attractive forces are stronger for ionic substances than for molecular ones, so BaCl2 should have the highest boiling point. The intermolecular forces of the remaining substances depend on molecular weight, polarity, and hydrogen bonding. The molecular weights are H2 (2), CO (28), HF (20), and Ne (20). The boiling point of H2 should be the lowest because it is nonpolar and has the lowest molecular weight. The molecular weights of CO, HF, and Ne are similar. Because HF can hydrogen bond, however, it should have the highest boiling point of the three. Next is CO, which is slightly polar and has the highest molecular weight. Finally, Ne, which is nonpolar, should have the lowest boiling point of these three. The predicted order of boiling points is, therefore,
H2 < Ne < CO < HF < BaCl2
Check The boiling points reported in the literature are H2 (20 K), Ne (27 K), CO (83 K), HF (293 K), and BaCl2 (1813 K)—in agreement with our predictions.
PRACTICE EXERCISE
(a) Identify the intermolecular attractions present in the following substances, and (b) select the substance with the highest boiling point: CH3CH3, CH3OH, and CH3CH2OH.
Answers: (a) CH3CH3 has only dispersion forces, whereas the other two substances have both dispersion forces and hydrogen bonds, (b) CH3CH2OH
 CHEMISTRY PUT TO WORK
CHEMISTRY PUT TO WORK
Ionic Liquids
The strong electrostatic attractions between cations and anions are responsible for the fact that most ionic compounds are solids at room temperature, with high melting and boiling points. However, the melting point of an ionic compound can be low if the ionic charges are not too high and the cation–anion distance is sufficiently large. For example, the melting point of NH4NO3, where both cation and anion are larger polyatomic ions, is 170 °C. If the ammonium cation is replaced by the even larger ethylammonium cation, CH3CH2NH3+, the melting point drops to 12 °C, making ethylammonium nitrate a liquid at room temperature! Ethylammonium nitrate is an example of an ionic liquid: a salt that is a liquid at room temperature.
Not only is CH3CH2NH3+ larger than NH4+ but also it is less symmetric. In general, the larger and more irregularly shaped the ions in an ionic substance, the better the chances of forming an ionic liquid. Although many cations form ionic liquids, one of the most popular is the 1-butyl-3-methylimidazolium cation (bmim+, ![]() FIGURE 11.16 and
FIGURE 11.16 and ![]() TABLE 11.3), which has two arms of different lengths coming off a five-atom central ring. This feature gives bmim+ an irregular shape, which makes it difficult for the molecules to pack together in a solid.
TABLE 11.3), which has two arms of different lengths coming off a five-atom central ring. This feature gives bmim+ an irregular shape, which makes it difficult for the molecules to pack together in a solid.
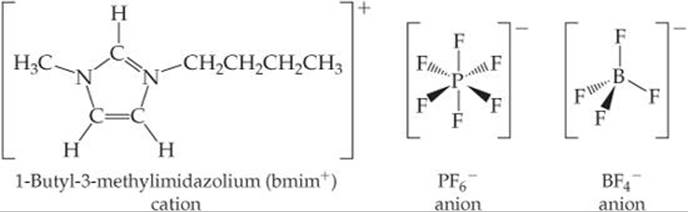
![]() FIGURE 11.16 Representative ions found in ionic liquids.
FIGURE 11.16 Representative ions found in ionic liquids.
TABLE 11.3 • Melting Point and Decomposition Temperature of Four 1-Butyl-3-methylimidazolium (bmim+) Salts
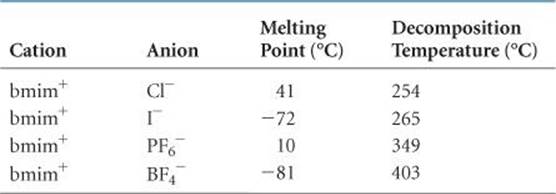
Common anions found in ionic liquids include the PF6–, BF4–, and halide ions.
Ionic liquids have properties that are attractive for some applications. Unlike most molecular liquids, they tend to have a very low vapor pressure. Because they are nonvolatile (that is, they don't evaporate), they tend to be nonflammable and remain in the liquid state at temperatures up to 673 K. Most molecular substances are liquids only at much lower temperatures, for example, 373 K or less in many cases (see Table 11.2). Because they are good solvents for a wide range of inorganic, organic, and polymeric substances, ionic liquids can be used for a variety of reactions and separations. These properties make them attractive replacements for volatile organic solvents in many industrial processes. Relative to traditional organic solvents, ionic liquids offer the promise of reduced volumes, safer handling, and easier reuse. For these reasons and others, there is considerable excitement about the promise of ionic liquids for reducing the environmental impact of industrial chemical processes.
RELATED EXERCISES: 11.31, 11.32, 11.81