CHEMISTRY THE CENTRAL SCIENCE
12 SOLIDS AND MODERN MATERIALS

THE HARD DRIVE OF A COMPUTER is made from an extremely smooth glass disc coated with a thin layer of a magnetic alloy of cobalt. To store and retrieve information, the read/write head must glide over the disc at a height of ~1 μm (less than 1/200 the width of a human hair) while the disk moves at speeds in excess of 7000 rpm. Devices such as this would not be possible without advanced solid-state materials.
WHAT'S HEAD
12.1 CLASSIFICATIONS OF SOLIDS
We see that solids can be classified according to the types of bonding interactions that hold the atoms together. This classification helps us make general predictions about the properties of solids.
12.2 STRUCTURES OF SOLIDS
We learn that in crystalline solids the atoms are arranged in an orderly, repeating pattern but in amorphous solids this order is missing. We learn about lattices and unit cells, which define the repeating patterns that characterize crystalline solids.
12.3 METALLIC SOLIDS
We examine the properties and structures of metals. We learn that many metals have structures in which the atoms pack together as closely as possible. We examine various types of alloys, materials that contain more than one element and display the characteristic properties of a metal.
12.4 METALLIC BONDING
We take a closer look at metallic bonding and how it is responsible for the properties of metals, in terms of two models—the electron-sea model and the molecular-orbital model. We learn how overlap of atomic orbitals gives rise to bands in metals.
12.5 IONIC SOLIDS
We examine the structures and properties of solids held together by the mutual attractions between cations and anions. We learn how the structures of ionic solids depend on the relative sizes of the ions and their stoichiometry.
12.6 MOLECULAR SOLIDS
We take a brief look at the solids that form when molecules are held together by weak intermolecular forces.
12.7 COVALENT-NETWORK SOLIDS
We learn about solids in which the atoms are held together by extended networks of covalent bonds. We learn how the electronic structure and properties of semiconductors differ from those of metals.
12.8 POLYMERIC SOLIDS
We investigate polymers—long chainlike molecules in which the motif of a small molecule is repeated many times over. We see how both molecular shape and interactions between polymer chains affect the physical properties of polymers.
12.9 NANOMATERIALS
We learn how the physical and chemical properties of materials change when their crystals become very small. These effects begin to occur when materials have sizes on the order of 1–100 nm. We explore lower-dimensional forms of carbon—fullerenes, carbon nanotubes, and graphene.
MODERN DEVICES LIKE COMPUTERS and cell phones are built from solids with very specific physical properties. For example, the integrated circuit that is at the heart of many electronic devices is built from semiconductors like silicon, metals like copper, and insulators like hafnium oxide. Hard drives, which store information in computers and other devices, consist of a thin layer of a magnetic alloy deposited on glass substrate.
Scientists and engineers turn almost exclusively to solids for materials used in many other technologies: alloys for magnets and airplane turbines, semiconductors for solar cells and light-emitting diodes, polymers for packaging and biomedical applications. Chemists have contributed to the discovery and development of new materials either by inventing new substances or by developing the means for processing natural materials to form substances that have specific electrical, magnetic, optical, or mechanical properties. In this chapter, we explore the structures and properties of solids. As we do so, we will examine some of the solid materials used in modern technology.
12.1 CLASSIFICATIONS OF SOLIDS
Solids can be as hard as diamond or as soft as wax. Some readily conduct electricity, whereas others do not. The shapes of some solids can easily be manipulated, while others are brittle and resistant to any change in shape. The physical properties as well as the structures of solids are dictated by the types of bonds that hold the atoms in place. We can classify solids according to those forces (![]() FIGURE 12.1).
FIGURE 12.1).
Metallic solids are held together by a delocalized “sea” of collectively shared valence electrons. This form of bonding allows metals to conduct electricity. It is also responsible for the fact that most metals are relatively strong without being brittle. Ionic solids are held together by the mutual attraction between cations and anions. Differences between ionic and metallic bonding make the electrical and mechanical properties of ionic solids very different from those of metals. Covalent-network solids are held together by an extended network of covalent bonds. This type of bonding can result in materials that are extremely hard, like diamond, and it is also responsible for the unique properties of semiconductors. Molecular solids are held together by the intermolecular forces we studied in Chapter 11: dispersion forces, dipole-dipole interactions, and hydrogen bonds. Because these forces are relatively weak, molecular solids tend to be soft and have low melting points.
We will also consider two classes of solids that do not fall neatly into the preceding categories: polymers and nanomaterials. Polymers contain long chains of atoms, where the atoms within a given chain are connected by covalent bonds and adjacent chains held to one another largely by weaker intermolecular forces. Polymers are normally stronger and have higher melting points than molecular solids, and they are more flexible than metallic, ionic, or covalent-network solids. Nanomaterials are solids in which the dimensions of individual crystals have been reduced to the order of 1-100 nm. As we will see, the properties of conventional materials change when their crystals become this small.
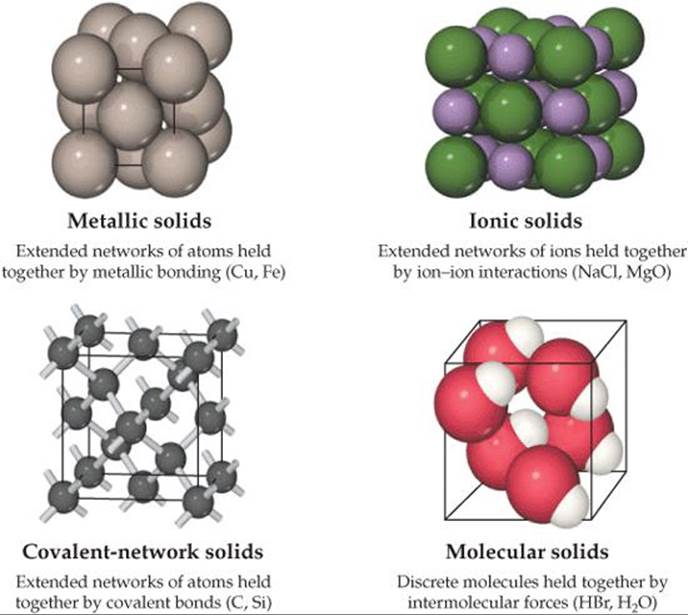
![]() FIGURE 12.1 Classifications of solids according to predominant bonding type.
FIGURE 12.1 Classifications of solids according to predominant bonding type.