CHEMISTRY THE CENTRAL SCIENCE
2 ATOMS, MOLECULES, AND IONS
2.3 THE MODERN VIEW OF ATOMIC STRUCTURE
Since Rutherford's time, as physicists have learned more and more about atomic nuclei, the list of particles that make up nuclei has grown and continues to increase. As chemists, however, we can take a simple view of the atom because only three subatomic particles—the proton, neutron, and electron—have a bearing on chemical behavior.
As noted earlier, the charge of an electron is –1.602 × 10–19 C. That of a proton is equal in magnitude, +1.602 × 10–19 C. The quantity 1.602 × 10–19 C is called the electronic charge. For convenience, the charges of atomic and subatomic particles are usually expressed as multiples of this charge rather than in coulombs. Thus, the charge of the electron is 1- and that of the proton is 1+. Neutrons are electrically neutral (which is how they received their name). Every atom has an equal number of electrons and protons, so atoms have no net electrical charge.
Protons and neutrons reside in the tiny nucleus of the atom. The vast majority of an atom's volume is the space in which the electrons reside (![]() FIGURE 2.11). The electrons are attracted to the protons in the nucleus by the electrostatic force that exists between particles of opposite electrical charge. In later chapters we will see that the strength of the attractive forces between electrons and nuclei can be used to explain many of the differences among different elements.
FIGURE 2.11). The electrons are attracted to the protons in the nucleus by the electrostatic force that exists between particles of opposite electrical charge. In later chapters we will see that the strength of the attractive forces between electrons and nuclei can be used to explain many of the differences among different elements.
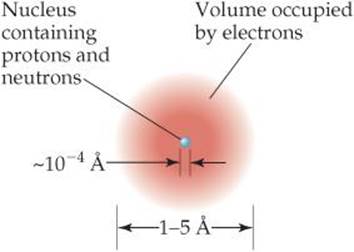
![]() Figure 2.11 The structure of the atom. A cloud of rapidly moving electrons occupies most of the volume of the atom. The nucleus occupies a tiny region at the center of the atom and is composed of the protons and neutrons. The nucleus contains virtually all the mass of the atom.
Figure 2.11 The structure of the atom. A cloud of rapidly moving electrons occupies most of the volume of the atom. The nucleus occupies a tiny region at the center of the atom and is composed of the protons and neutrons. The nucleus contains virtually all the mass of the atom.
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
a. If an atom has 15 protons, how many electrons does it have?
b. Where do the protons reside in an atom?
Atoms have extremely small masses. The mass of the heaviest known atom, for example, is approximately 4 × 10–22 g. Because it would be cumbersome to express such small masses in grams, we use the atomic mass unit (amu),* where 1 amu = 1.66054 × 10–24 g. A proton has a mass of 1.0073 amu, a neutron 1.0087 amu, and an electron 5.486 × 10–4 amu (![]() TABLE 2.1). Because it takes 1836 electrons to equal the mass of one proton or one neutron, the nucleus contains most of the mass of an atom.
TABLE 2.1). Because it takes 1836 electrons to equal the mass of one proton or one neutron, the nucleus contains most of the mass of an atom.
Most atoms have diameters between 1 × 10–10 m and 5 × 10–10 m. A convenient non-SI unit of length used for atomic dimensions is the angstrom (Å), where 1 Å = 1 × 10–10 m. Thus, atoms have diameters of approximately 1 – 5 Å. The diameter of a chlorine atom, for example, is 200 pm, or 2.0 Å.
SAMPLE EXERCISE 2.1 Atomic Size
The diameter of a US dime is 17.9 mm, and the diameter of a silver atom is 2.88 Å. How many silver atoms could be arranged side by side across the diameter of a dime?
SOLUTION
The unknown is the number of silver (Ag) atoms. Using the relationship 1 Ag atom = 2.88 Å as a conversion factor relating number of atoms and distance, we start with the diameter of the dime, first converting this distance into angstroms and then using the diameter of the Ag atom to convert distance to number of Ag atoms:
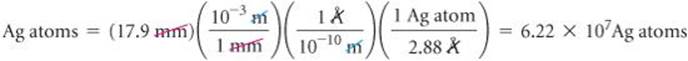
That is, 62.2 million silver atoms could sit side by side across a dime!
PRACTICE EXERCISE
The diameter of a carbon atom is 1.54 Å. (a) Express this diameter in picometers. (b) How many carbon atoms could be aligned side by side across the width of a pencil line that is 0.20 mm wide?
Answers: (a) 154 pm, (b) 1.3 × 106 C atoms
The diameter of an atomic nucleus is approximately 10–4 Å, only a small fraction of the diameter of the atom as a whole. You can appreciate the relative sizes of the atom and its nucleus by imagining that if the hydrogen atom were as large as a football stadium, the nucleus would be the size of a small marble. Because the tiny nucleus carries most of the mass of the atom in such a small volume, it has an incredibly high density—on the order of 1013–1014 g/cm3. A matchbox full of material of such density would weigh over 2.5 billion tons!
TABLE 2.1 • Comparison of the Proton, Neutron, and Electron

 A CLOSER LOOK
A CLOSER LOOK
BASIC FORCES
Four basic forces are known in nature: (1) gravitational, (2) electromagnetic, (3) strong nuclear, and (4) weak nuclear. Gravitational forces are attractive forces that act between all objects in proportion to their masses. Gravitational forces between atoms or between subatomic particles are so small that they are of no chemical significance.
Electromagnetic forces are attractive or repulsive forces that act between either electrically charged or magnetic objects. Electric forces are important in understanding the chemical behavior of atoms. The magnitude of the electric force between two charged particles is given byCoulomb's law: F = kQ1Q2/d2, where Q1 and Q2 are the magnitudes of the charges on the two particles, d is the distance between their centers, and k is a constant determined by the units for Q and d. A negative value for the force indicates attraction, whereas a positive value indicates repulsion.
All nuclei except those of hydrogen atoms contain two or more protons. Because like charges repel, electrical repulsion would cause the protons to fly apart if the strong nuclear force did not keep them together. This force acts between subatomic particles, as in the nucleus. At this distance, the attractive strong nuclear force is stronger than the positive-positive repulsive electric force and holds the nucleus together.
The weak nuclear force is weaker than the electric force but stronger than the gravitational force. We are aware of its existence only because it shows itself in certain types of radioactivity.
RELATED EXERCISE: 2.88
An illustration of the atom that incorporates the features we have just discussed is shown in Figure 2.11. The electrons play the major role in chemical reactions. The significance of representing the region containing the electrons as an indistinct cloud will become clear in later chapters when we consider the energies and spatial arrangements of the electrons.
Atomic Numbers, Mass Numbers, and Isotopes
What makes an atom of one element different from an atom of another element is that the atoms of each element have a characteristic number of protons. Indeed, the number of protons in an atom of any particular element is called that element's atomic number. Because an atom has no net electrical charge, the number of electrons it contains must equal the number of protons. All atoms of carbon, for example, have six protons and six electrons, whereas all atoms of oxygen have eight protons and eight electrons. Thus, carbon has atomic number 6, and oxygen has atomic number 8. The atomic number of each element is listed with the name and symbol of the element on the inside front cover of the text.
Atoms of a given element can differ in the number of neutrons they contain and, consequently, in mass. For example, most atoms of carbon have six neutrons, although some have more and some have less. The symbol ![]() (read “carbon twelve,” carbon-12) represents the carbon atom containing six protons and six neutrons. The atomic number is shown by the subscript; the superscript, called the mass number, is the number of protons plus neutrons in the atom:
(read “carbon twelve,” carbon-12) represents the carbon atom containing six protons and six neutrons. The atomic number is shown by the subscript; the superscript, called the mass number, is the number of protons plus neutrons in the atom:
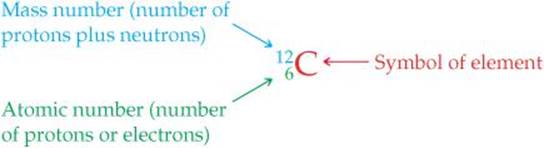
Because all atoms of a given element have the same atomic number, the subscript is redundant and is often omitted. Thus, the symbol for carbon-12 can be represented simply as 12C. As one more example of this notation, carbon atoms that contain six protons and eight neutrons have mass number 14, are represented as ![]() or 14C, and are referred to as carbon-14.
or 14C, and are referred to as carbon-14.
TABLE 2.2 • Some Isotopes of Carbon*
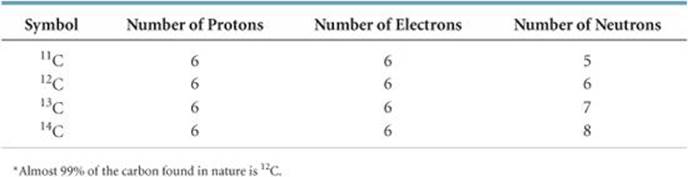
Atoms with identical atomic numbers but different mass numbers (that is, same number of protons but different numbers of neutrons) are called isotopes of one another. Several isotopes of carbon are listed in ![]() TABLE 2.2. We will generally use the notation with superscripts only when referring to a particular isotope of an element.
TABLE 2.2. We will generally use the notation with superscripts only when referring to a particular isotope of an element.
SAMPLE EXERCISE 2.2 Determining the Number of Subatomic Particles in Atoms
How many protons, neutrons, and electrons are in (a) an atom of 197Au, (b) an atom of strontium-90?
SOLUTION
(a) The superscript 197 is the mass number (protons + neutrons). According to the list of elements given on the inside front cover, gold has atomic number 79. Consequently, an atom of 197Au has 79 protons, 79 electrons, and 197 – 79 = 118 neutrons. (b) The atomic number of strontium (listed on inside front cover) is 38. Thus, all atoms of this element have 38 protons and 38 electrons. The strontium-90 isotope has 90 – 38 = 52 neutrons.
PRACTICE EXERCISE
How many protons, neutrons, and electrons are in (a) a 138Ba atom, (b) an atom of phosphorus-31?
Answer: (a) 56 protons, 56 electrons, and 82 neutrons, (b) 15 protons, 15 electrons, and 16 neutrons
SAMPLE EXERCISE 2.3 Writing Symbols for Atoms
Magnesium has three isotopes with mass numbers 24, 25, and 26. (a) Write the complete chemical symbol (superscript and subscript) for each. (b) How many neutrons are in an atom of each isotope?
SOLUTION
(a) Magnesium has atomic number 12, so all atoms of magnesium contain 12 protons and 12 electrons. The three isotopes are therefore represented by ![]() ,
, ![]() , and
, and ![]() . (b) The number of neutrons in each isotope is the mass number minus the number of protons. The numbers of neutrons in an atom of each isotope are therefore 12, 13, and 14, respectively.
. (b) The number of neutrons in each isotope is the mass number minus the number of protons. The numbers of neutrons in an atom of each isotope are therefore 12, 13, and 14, respectively.
PRACTICE EXERCISE
Give the complete chemical symbol for the atom that contains 82 protons, 82 electrons, and 126 neutrons.
Answer: ![]()