CHEMISTRY THE CENTRAL SCIENCE
12 SOLIDS AND MODERN MATERIALS
12.8 POLYMERIC SOLIDS
In nature we find many substances of very high molecular weight, running into millions of amu, that make up much of the structure of living organisms and tissues. Some examples are starch and cellulose, which abound in plants, as well as proteins, which are found in both plants and animals. In 1827 Jons Jakob Berzelius coined the word polymer (from the Greek polys, “many,” and meros, “parts”) to denote molecular substances of high molecular weight formed by the polymerization (joining together) of monomers, molecules with low molecular weight.
 CHEMISTRY PUT TO WORK
CHEMISTRY PUT TO WORK
Solid-State Lighting
Artificial lighting is so widespread we take it for granted. As described in Chapter 1, major savings in energy would be realized if incandescent lights can be replaced by light-emitting diodes (LEDs) (Section 1.4, “Chemistry Put to Work”). Because LEDs are made from semiconductors, this is an appropriate place to take a closer look at the operation of an LED.
The heart of an LED is a p-n diode, which is formed by bringing an n-type semiconductor in contact with a p-type semiconductor. In the junction where they meet there are very few electrons or holes to carry the charge and the conductivity decreases. When an appropriate voltage is applied, it drives electrons from the conduction band of the n-doped side into the junction, where they meet holes that are pushed in from the valence band of the p-doped side. The electrons fall into the empty holes, and their energy is converted into light whose photons have energy equal to the band gap (![]() FIGURE 12.33). In this way electrical energy is converted into optical energy.
FIGURE 12.33). In this way electrical energy is converted into optical energy.
Because the wavelength of light that is emitted depends on the band gap of the semiconductor, the color of light produced by the LED can be controlled by appropriate choice of semiconductor. Most red LEDs are made of a mixture of GaP and GaAs. The band gap of GaP is 2.26 eV (3.62 × 10-19 J), which corresponds to a green photon with a wavelength of 549 nm, while GaAs has a band gap of 1.43 eV (2.29 × 10-19 J), which corresponds to an infrared photon with a wavelength of 867 nm. ![]() (Section 6.1 and 6.2) By forming solid solutions of these two compounds, with stoichiometries of GaP1–xAsx, the band gap can be adjusted to any intermediate value. Thus, GaP1–xAsx is the solid solution of choice for red, orange, and yellow LEDs. Green LEDs are made from mixtures of GaP and AlP (Eg = 2.43eV, λ = 510nm).
(Section 6.1 and 6.2) By forming solid solutions of these two compounds, with stoichiometries of GaP1–xAsx, the band gap can be adjusted to any intermediate value. Thus, GaP1–xAsx is the solid solution of choice for red, orange, and yellow LEDs. Green LEDs are made from mixtures of GaP and AlP (Eg = 2.43eV, λ = 510nm).
Red LEDs have been in the market for decades, but to make white light an efficient blue LED was needed. The first prototype bright blue LED was demonstrated in Japan in 1993. In 2006, only 13 years later, over $4 billion worth of blue LEDs were sold worldwide. The blue LEDs are based on combinations of GaN (Eg = 3.4eV, λ = 365 nm) and InN (Eg = 2.4eV, λ = 517 nm). With the availability of blue LEDs there are various strategies for making white LEDs. In some cases, light is combined from blue, green, and red LEDs. More commonly a blue LED is coated with a phosphor, a material that converts some of the blue light into yellow light. In either case the combined colors appear white to the eye. Some examples of different color LEDs are shown in ![]() FIGURE 12.34.
FIGURE 12.34.
RELATED EXERCISES: 12.71, 12.72, 12.73, 12.74
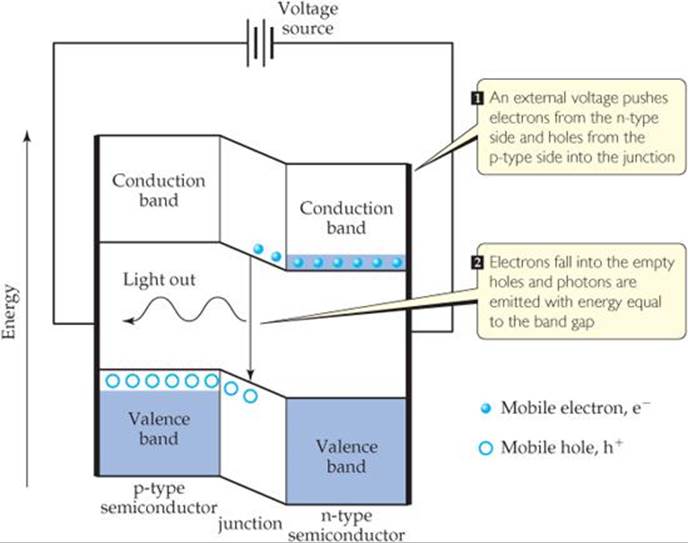
![]() FIGURE 12.33 Light-emitting diodes. The heart of a light-emitting diode is a p-n junction in which an applied voltage drives electrons and holes to combine and give off light.
FIGURE 12.33 Light-emitting diodes. The heart of a light-emitting diode is a p-n junction in which an applied voltage drives electrons and holes to combine and give off light.

![]() FIGURE 12.34 Different colors of light-emitting diodes.
FIGURE 12.34 Different colors of light-emitting diodes.
Historically natural polymers, such as wool, leather, silk, and natural rubber, were processed into usable materials. During the past 70 years or so, chemists have learned to form synthetic polymers by polymerizing monomers through controlled chemical reactions. A great many of these synthetic polymers have a backbone of carbon–carbon bonds because carbon atoms have an exceptional ability to form strong stable bonds with one another.
Plastics are materials that can be formed into various shapes, usually by the application of heat and pressure. Thermoplastic materials can be reshaped. For example, plastic milk containers are made from the polymer polyethylene. These containers can be melted down and the polymer recycled for some other use. In contrast, a thermosetting plastic is shaped through irreversible chemical processes and, therefore, cannot be reshaped readily. An elastomer is a material that exhibits rubbery or elastic behavior. When subjected to stretching or bending, an elastomer regains its original shape upon removal of the distorting force, if it has not been distorted beyond some elastic limit. Rubber is the most familiar example of an elastomer.
Some polymers, such as nylon and polyesters, both of which are thermosetting plastics, can be formed into fibers that, like hair, are very long relative to their cross-sectional area. These fibers can be woven into fabrics and cords and fashioned into clothing, tire cord, and other useful objects.
Making Polymers
The simplest example of a polymerization reaction is the formation of polyethylene from ethylene molecules (![]() FIGURE 12.35). In this reaction, the double bond in each ethylene molecule “opens up,” and two of the electrons originally in this bond are used to form new C—C single bonds with two other ethylene molecules. This type of polymerization, in which monomers are coupled through their multiple bonds, is called addition polymerization.
FIGURE 12.35). In this reaction, the double bond in each ethylene molecule “opens up,” and two of the electrons originally in this bond are used to form new C—C single bonds with two other ethylene molecules. This type of polymerization, in which monomers are coupled through their multiple bonds, is called addition polymerization.
We can write the equation for the polymerization reaction as follows:
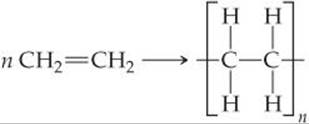
Here n represents the large number—ranging from hundreds to many thousands—of monomer molecules (ethylene in this case) that react to form one polymer molecule. Within the polymer, a repeat unit (the unit shown in brackets in equation above) appears over and over along the entire chain. The ends of the chain are capped by carbon-hydrogen bonds or by some other bond, so that the end carbons have four bonds.
Polyethylene is an important material; its annual production exceeds 170 billion pounds each year. Although its composition is simple, the polymer is not easy to make. The right manufacturing conditions were identified only after many years of research. Today many forms of polyethylene, varying widely in physical properties, are known.
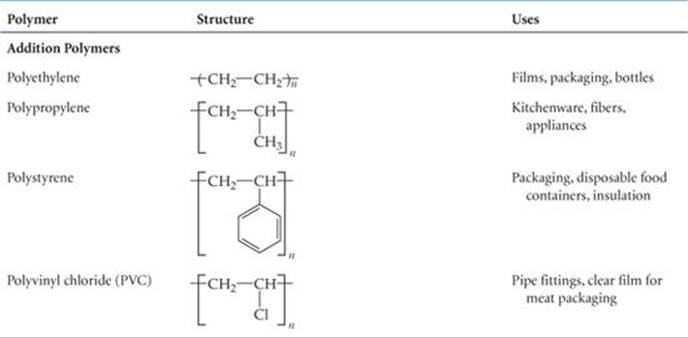
Polymers of other chemical compositions provide still greater variety in physical and chemical properties. ![]() TABLE 12.5 lists several other common polymers obtained by addition polymerization.
TABLE 12.5 lists several other common polymers obtained by addition polymerization.
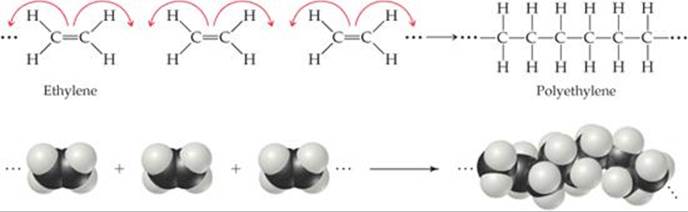
![]() FIGURE 12.35 The polymerization of ethylene monomers to make the polymer polyethylene.
FIGURE 12.35 The polymerization of ethylene monomers to make the polymer polyethylene.
TABLE 12.5 • Polymers of Commercial Importance
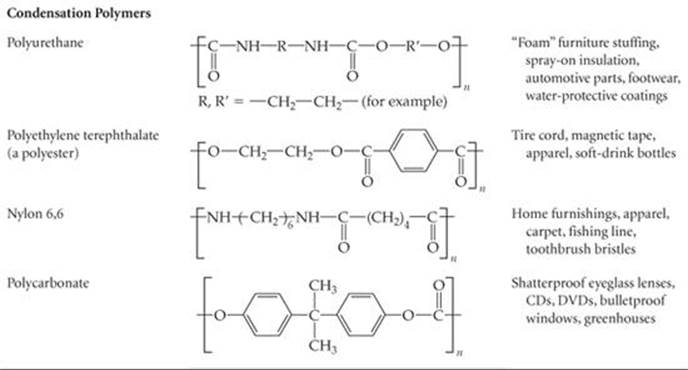
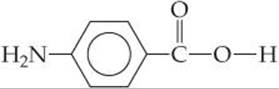
A second general reaction used to synthesize commercially important polymers is condensation polymerization. In a condensation reaction two molecules are joined to form a larger molecule by elimination of a small molecule, such as H2O. For example, an amine (a compound containing—NH2) reacts with a carboxylic acid (a compound containing—COOH) to form a bond between N and C plus an H2O molecule (![]() FIGURE 12.37).
FIGURE 12.37).
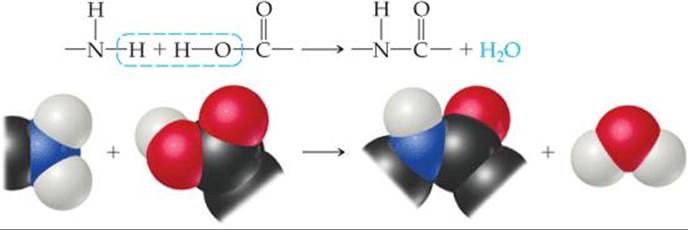
![]() FIGURE 12.37 A condensation polymerization.
FIGURE 12.37 A condensation polymerization.
 CHEMISTRY PUT TO WORK
CHEMISTRY PUT TO WORK
Recycling Plastics
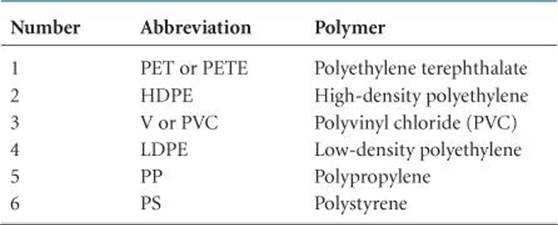
If you look at the bottom of a plastic container, you are likely to see a recycle symbol containing a number, as shown in ![]() FIGURE 12.36. The number and the letter abbreviation below it indicate the kind of polymer from which the container is made, as summarized in
FIGURE 12.36. The number and the letter abbreviation below it indicate the kind of polymer from which the container is made, as summarized in ![]() TABLE 12.6. (The chemical structures of these polymers are shown in Table 12.5.) These symbols make it possible to sort containers by composition. In general, the lower the number, the greater the ease with which the material can be recycled.
TABLE 12.6. (The chemical structures of these polymers are shown in Table 12.5.) These symbols make it possible to sort containers by composition. In general, the lower the number, the greater the ease with which the material can be recycled.
TABLE 12.6 • Categories Used for Recycling Polymeric Materials in the United States

![]() FIGURE 12.36 Recycling symbols. Most plastic containers manufactured today carry a recycling symbol indicating the type of polymer used to make the container and the polymer's suitability for recycling.
FIGURE 12.36 Recycling symbols. Most plastic containers manufactured today carry a recycling symbol indicating the type of polymer used to make the container and the polymer's suitability for recycling.
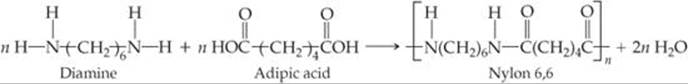
![]() FIGURE 12.38 The formation of the copolymer nylon 6,6.
FIGURE 12.38 The formation of the copolymer nylon 6,6.
Polymers formed from two different monomers are called copolymers. In the formation of many nylons, a diamine, a compound with an —NH2 group at each end, is reacted with a diacid, a compound with a —COOH group at each end. For example, the copolymer nylon 6,6 is formed when a diamine that has six carbon atoms and an amino group on each end is reacted with adipic acid, which also has six carbon atoms (![]() FIGURE 12.38). A condensation reaction occurs on each end of the diamine and the acid. The components of H2O are split out, and N—C bonds are formed between molecules.
FIGURE 12.38). A condensation reaction occurs on each end of the diamine and the acid. The components of H2O are split out, and N—C bonds are formed between molecules.
Table 12.5 lists nylon 6,6 and some other common polymers obtained by condensation polymerization. Notice that these polymers have backbones containing N or O atoms as well as C atoms.
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
Is this molecule a better starting material for an addition polymer or a condensation polymer?
Structure and Physical Properties of Polymers
The simple structural formulas given for polyethylene and other polymers are deceptive. Because four bonds surround each carbon atom in polyethylene, the atoms are arranged in a tetrahedral fashion, so that the chain is not straight as we have depicted it. Furthermore, the atoms are relatively free to rotate around the C—C single bonds. Rather than being straight and rigid, therefore, the chains are flexible, folding readily (![]() FIGURE 12.39). The flexibility in the molecular chains causes any material made of this polymer to be very flexible.
FIGURE 12.39). The flexibility in the molecular chains causes any material made of this polymer to be very flexible.
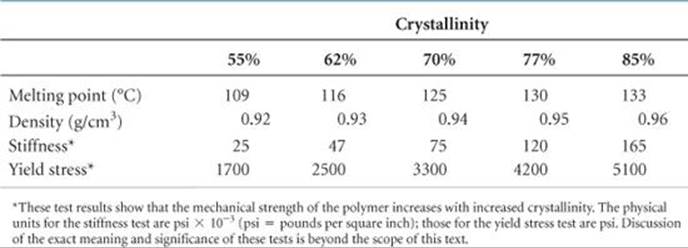
Both synthetic and natural polymers commonly consist of a collection of macromolecules (large molecules) of different molecular weights. Depending on the conditions of formation, the molecular weights may be distributed over a wide range or may be closely clustered around an average value. In part because of this distribution in molecular weights, polymers are largely amorphous (noncrystalline) materials. Rather than exhibiting a well-defined crystalline phase with a sharp melting point, polymers soften over a range of temperatures. They may, however, possess short-range order in some regions of the solid, with chains lined up in regular arrays as shown in ![]() FIGURE 12.40. The extent of such ordering is indicated by the degree of crystallinity of the polymer. Mechanical stretching or pulling to align the chains as the molten polymer is drawn through small holes can frequently enhance the crystallinity of a polymer. Inter-molecular forces between the polymer chains hold the chains together in the ordered crystalline regions, making the polymer denser, harder, less soluble, and more resistant to heat.
FIGURE 12.40. The extent of such ordering is indicated by the degree of crystallinity of the polymer. Mechanical stretching or pulling to align the chains as the molten polymer is drawn through small holes can frequently enhance the crystallinity of a polymer. Inter-molecular forces between the polymer chains hold the chains together in the ordered crystalline regions, making the polymer denser, harder, less soluble, and more resistant to heat. ![]() TABLE 12.7 shows how the properties of polyethylene change as the degree of crystallinity increases.
TABLE 12.7 shows how the properties of polyethylene change as the degree of crystallinity increases.
The linear structure of polyethylene is conducive to intermolecular interactions that lead to crystallinity. However, the degree of crystallinity in polyethylene strongly depends on the average molecular weight. Polymerization results in a mixture of macro-molecules with varying values of n and, hence, varying molecular weights. Low-density polyethylene (LDPE), used in forming films and sheets, has an average molecular weight in the range of 104 amu and has substantial chain branching. That is, there are side chains off the main chain of the polymer. These side chains inhibit the formation of crystalline regions, reducing the density of the material. High-density polyethylene (HDPE), used to form bottles, drums, and pipes, has an average molecular weight in the range of 106 amu. This form has fewer side chains and thus a higher degree of crystallinity.
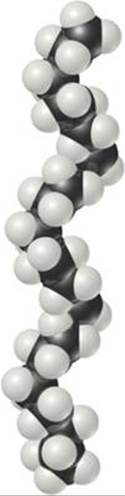
![]() FIGURE 12.39 A segment of a polyethylene chain. This segment consists of 28 carbon atoms. In commercial polyethylenes, the chain lengths range from about 103 to 105 CH2 units.
FIGURE 12.39 A segment of a polyethylene chain. This segment consists of 28 carbon atoms. In commercial polyethylenes, the chain lengths range from about 103 to 105 CH2 units.
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
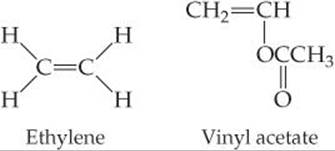
In copolymers made of ethylene and vinyl acetate monomers, melting point and degree of crystallinity decrease as the percentage of vinyl acetate increases. Suggest an explanation.
Polymers can be made stiffer by introducing chemical bonds between chains. Forming bonds between chains is called cross-linking (![]() FIGURE 12.41). The greater the number of cross-links, the more rigid the polymer. Whereas thermoplastic materials consist of independent polymer chains, thermosetting ones become cross-linked when heated; the cross-links allow them to hold their shapes.
FIGURE 12.41). The greater the number of cross-links, the more rigid the polymer. Whereas thermoplastic materials consist of independent polymer chains, thermosetting ones become cross-linked when heated; the cross-links allow them to hold their shapes.
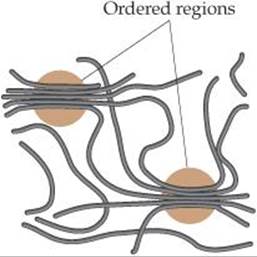
![]() FIGURE 12.40 Interactions between polymer chains. In the circled regions, the forces that operate between adjacent segments of the chains lead to ordering analogous to the ordering in crystals, though less regular.
FIGURE 12.40 Interactions between polymer chains. In the circled regions, the forces that operate between adjacent segments of the chains lead to ordering analogous to the ordering in crystals, though less regular.
TABLE 12.7 • Properties of Polyethylene as a Function of Crystallinity

![]() FIGURE 12.41 Cross-linking of polymer chains. The cross-linking groups (red) constrain the relative motions of the polymer chains, making the material harder and less flexible than when the cross-links are not present.
FIGURE 12.41 Cross-linking of polymer chains. The cross-linking groups (red) constrain the relative motions of the polymer chains, making the material harder and less flexible than when the cross-links are not present.
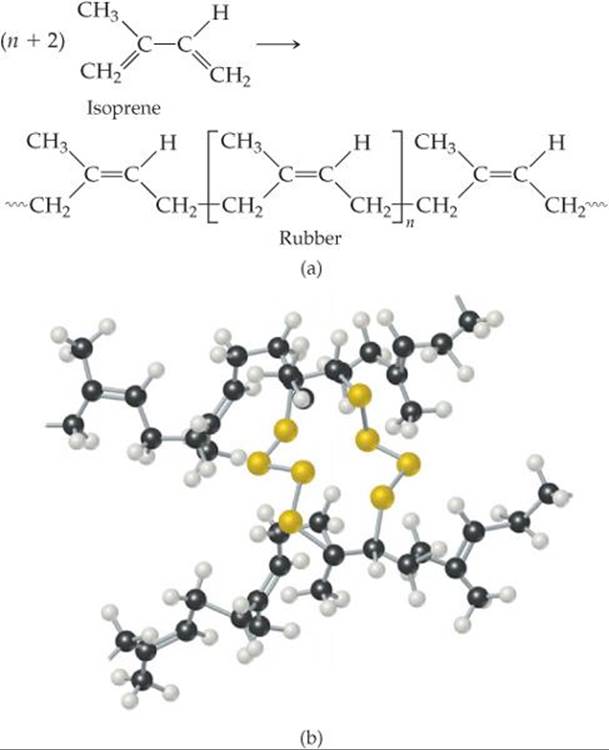
![]() FIGURE 12.42 Vulcanization of natural rubber. (a) Formation of polymeric natural rubber from the monomer isoprene. (b) Adding sulfur to rubber creates sulfur-atom links between chains. These links form as the carbon-carbon double bonds in the natural rubber polymer open up.
FIGURE 12.42 Vulcanization of natural rubber. (a) Formation of polymeric natural rubber from the monomer isoprene. (b) Adding sulfur to rubber creates sulfur-atom links between chains. These links form as the carbon-carbon double bonds in the natural rubber polymer open up.
An important example of cross-linking is the vulcanization of natural rubber, a process discovered by Charles Goodyear in 1839. Natural rubber is formed from a liquid resin derived from the inner bark of the Hevea brasiliensis tree. Chemically, it is a polymer of isoprene, C5H8 (![]() FIGURE 12.42). Because rotation about the carbon-carbon double bond does not readily occur, the orientation of the groups bound to the carbons is rigid. In natural rubber, the chain extensions are on the same side of the double bond, as shown in Figure 12.42(a).
FIGURE 12.42). Because rotation about the carbon-carbon double bond does not readily occur, the orientation of the groups bound to the carbons is rigid. In natural rubber, the chain extensions are on the same side of the double bond, as shown in Figure 12.42(a).
Natural rubber is not a useful polymer because it is too soft and too chemically reactive. Goodyear accidentally discovered that adding sulfur and then heating the mixture makes the rubber harder and reduces its susceptibility to oxidation or other chemical attack. The sulfur changes rubber into a thermosetting polymer by cross-linking the polymer chains through reactions at some of the double bonds, as shown schematically in Figure 12.42(b). Cross-linking of about 5% of the double bonds creates a flexible, resilient rubber. When the rubber is stretched, the cross-links help prevent the chains from slipping, so that the rubber retains its elasticity. Because heating was an important step in his process, Goodyear named it after Vulcan, the Roman god of fire.