CHEMISTRY THE CENTRAL SCIENCE
13 PROPERTIES OF SOLUTIONS
13.6 COLLOIDS
Finely divided clay particles dispersed in water eventually settle out because of gravity. This happens because clay molecules, which consist of thousands or even millions of atoms, are much larger than most molecules. In contrast, the dispersed particles in a solution (ions in a salt solution, say, or glucose molecules in a sugar solution) are smaller. Between these extremes lie dispersed particles that are larger than typical molecules but not so large that the components of the mixture separate under the influence of gravity. These intermediate types of dispersions are called eithercolloidal dispersions or simply colloids. Colloids form the dividing line between solutions and heterogeneous mixtures. Like solutions, colloids can be gases, liquids, or solids. Examples of each are listed in ![]() TABLE 13.5.
TABLE 13.5.
Particle size can be used to classify a mixture as colloid or solution. Colloid particles range in diameter from 5 to 1000 nm; solute particles are smaller than 5 nm in diameter. A colloid particle may even consist of a single giant molecule. The hemoglobin molecule, for example, which carries oxygen in your blood, has molecular dimensions of 6.5 × 5.5 × 5.0 nm and a molar mass of 64,500 g/mol.
Although colloid particles may be so small that the dispersion appears uniform even under a microscope, they are large enough to scatter light. Consequently, most colloids appear cloudy or opaque unless they are very dilute. (Homogenized milk is a colloid.) Furthermore, because they scatter light, a light beam can be seen as it passes through a colloidal dispersion (![]() FIGURE 13.28). This scattering of light by colloidal particles, known as the Tyndall effect, makes it possible to see the light beam of an automobile on a dusty dirt road or the sunlight coming through a forest canopy
FIGURE 13.28). This scattering of light by colloidal particles, known as the Tyndall effect, makes it possible to see the light beam of an automobile on a dusty dirt road or the sunlight coming through a forest canopy ![]() FIGURE 13.29). Not all wavelengths are scattered to the same extent. Colors at the violet end of the visible spectrum are scattered more than those at the red end. As a result, brilliant red sunsets are seen when the sun is near the horizon and the air contains dust, smoke, or other particles of colloidal size.
FIGURE 13.29). Not all wavelengths are scattered to the same extent. Colors at the violet end of the visible spectrum are scattered more than those at the red end. As a result, brilliant red sunsets are seen when the sun is near the horizon and the air contains dust, smoke, or other particles of colloidal size.
TABLE 13.5 • Types of Colloids

![]() FIGURE 13.28 Tyndall effect in the laboratory. The glass on the right contains a colloidal dispersion; that on the left contains a solution.
FIGURE 13.28 Tyndall effect in the laboratory. The glass on the right contains a colloidal dispersion; that on the left contains a solution.
Hydrophilic and Hydrophobic Colloids
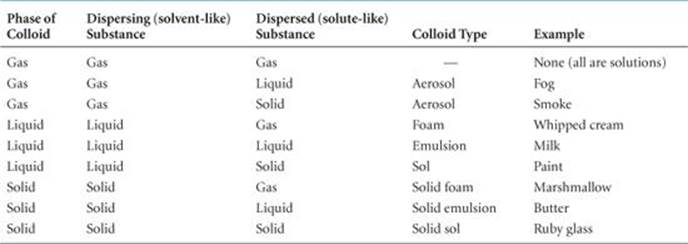
The most important colloids are those in which the dispersing medium is water. These colloids may be hydrophilic (water loving) or hydrophobic (water fearing). Hydrophilic colloids are most like the solutions that we have previously examined. In the human body the extremely large molecules that make up such important substances as enzymes and antibodies are kept in suspension by interaction with surrounding water molecules. A hydrophilic molecule folds in such a way that its hydrophobic groups are away from the water molecules, on the “inside” of the folded molecule, while its hydrophilic, polar groups are on the surface, interacting with the water molecules. The hydrophilic groups generally contain oxygen or nitrogen and often carry a charge (![]() FIGURE 13.30).
FIGURE 13.30).
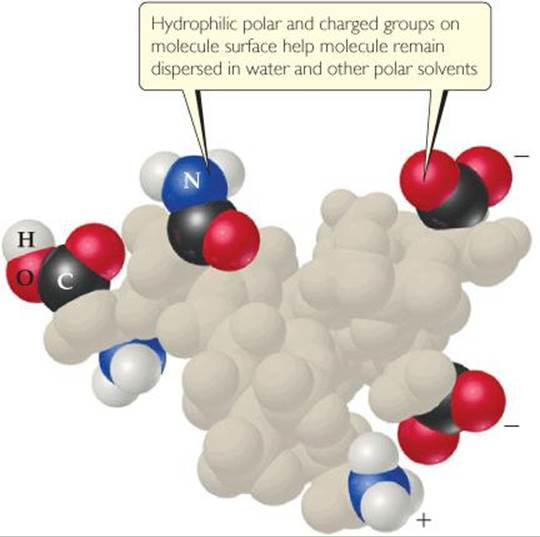
Hydrophobic colloids can be dispersed in water only if they are stabilized in some way. Otherwise, their natural lack of affinity for water causes them to separate from the water. One method of stabilization involves adsorbing ions on the surface of the hydrophobic particles (![]() FIGURE 13.31). (Adsorption means to adhere to a surface. It differs from absorption, which means to pass into the interior, as when a sponge absorbs water.) The adsorbed ions can interact with water, thereby stabilizing the colloid. At the same time, the repulsion between adsorbed ions on neighboring colloid particles keeps the particles from sticking together rather than dispersing in the water.
FIGURE 13.31). (Adsorption means to adhere to a surface. It differs from absorption, which means to pass into the interior, as when a sponge absorbs water.) The adsorbed ions can interact with water, thereby stabilizing the colloid. At the same time, the repulsion between adsorbed ions on neighboring colloid particles keeps the particles from sticking together rather than dispersing in the water.

![]() FIGURE 13.29 Tyndall effect in nature.
FIGURE 13.29 Tyndall effect in nature.
![]() GO FIGURE
GO FIGURE
What is the chemical composition of the groups that carry a negative charge?
![]() FIGURE 13.30 Hydrophilic colloidal particle. Examples of the hydrophilic groups that help to keep a giant molecule (macromolecule) suspended in water.
FIGURE 13.30 Hydrophilic colloidal particle. Examples of the hydrophilic groups that help to keep a giant molecule (macromolecule) suspended in water.
![]() FIGURE 13.31 Hydrophobic colloids stabilized by adsorbed anions.
FIGURE 13.31 Hydrophobic colloids stabilized by adsorbed anions.
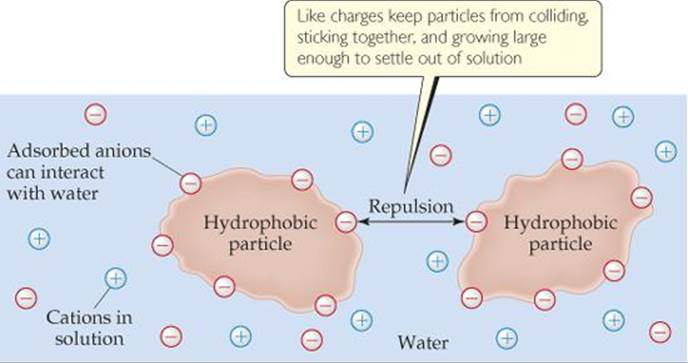
Hydrophobic colloids can also be stabilized by hydrophilic groups on their surfaces. Oil drops are hydrophobic, for example, so they do not remain suspended in water. Instead, they aggregate, forming an oil slick on the water surface. Sodium stearate (![]() FIGURE 13.32), or any similar substance having one end that is hydrophilic (either polar or charged) and one end that is hydrophobic (nonpolar), will stabilize a suspension of oil in water. Stabilization results from the interaction of the hydrophobic ends of the stearate ions with the oil drops and the hydrophilic ends with the water.
FIGURE 13.32), or any similar substance having one end that is hydrophilic (either polar or charged) and one end that is hydrophobic (nonpolar), will stabilize a suspension of oil in water. Stabilization results from the interaction of the hydrophobic ends of the stearate ions with the oil drops and the hydrophilic ends with the water.
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
Why don't oil drops stabilized by sodium stearate coagulate to form larger oil drops?
![]() GO FIGURE
GO FIGURE
Which kind of intermolecular force attracts the stearate ion to the oil drop?
![]() FIGURE 13.32 Stabilization of an emulsion of oil in water by stearate ions.
FIGURE 13.32 Stabilization of an emulsion of oil in water by stearate ions.
Colloid stabilization has an interesting application in the human digestive system. When fats in our diet reach the small intestine, a hormone causes the gallbladder to excrete a fluid called bile. Among the components of bile are compounds that have chemical structures similar to sodium stearate; that is, they have a hydrophilic (polar) end and a hydrophobic (nonpolar) end. These compounds emulsify the fats in the intestine and thus permit digestion and absorption of fat-soluble vitamins through the intestinal wall. The term emulsify means “to form an emulsion,” a suspension of one liquid in another, with milk being one example (Table 13.5). A substance that aids in the formation of an emulsion is called an emulsifying agent. If you read the labels on foods and other materials, you will find that a variety of chemicals are used as emulsifying agents. These chemicals typically have a hydrophilic end and a hydrophobic end.
Removal of Colloidal Particles
Colloidal particles frequently must be removed from a dispersing medium, as in the removal of smoke from smokestacks or butterfat from milk. Because colloidal particles are too small to be separated by simple filtration, they must be enlarged in a process called coagulation. The coagulated particles can then be separated by filtration or merely allowed to settle out of the dispersing medium.
Heating a colloidal dispersion or adding an electrolyte may bring about coagulation. Heating increases the particle motion and so the number of collisions. The particles get larger as they stick together after colliding. Addition of an electrolyte neutralizes the surface charges of the particles, thereby removing the electrostatic repulsions that prevent them from coming together. Wherever a river empties into the ocean, for example, the clay suspended in the river is deposited as a delta as it mixes with the electrolytes in the salt water.
A semipermeable membrane can be used to separate ions from colloidal particles because the ions can pass through the membrane but the colloidal particles cannot. This type of separation is known as dialysis and is used to purify blood in artificial kidney machines. Our kidneys normally remove waste products from blood. In a kidney machine, blood is circulated through a dialyzing tube immersed in a washing solution. The solution contains the same concentrations and kinds of ions as blood but no waste products. Dissolved wastes therefore dialyze out of the blood, but the large colloidal particles such as proteins do not.
 CHEMISTRY AND LIFE
CHEMISTRY AND LIFE
SICKLE-CELL ANEMIA
Our blood contains the complex protein hemoglobin, which carries oxygen from the lungs to other parts of the body. In the genetic disease known as sickle-cell anemia, hemoglobin molecules are abnormal and have a lower solubility, especially in their unoxygenated form. Consequently, as much as 85% of the hemoglobin in red blood cells crystallizes out of solution.
The cause of the insolubility is a structural change in one part of an amino acid. Normal hemoglobin molecules contain an amino acid that has a —CH2CH2COOH chain:
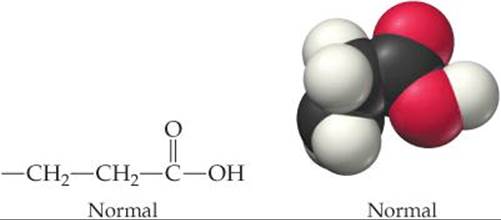
The polarity of the —COOH group contributes to the solubility of the hemoglobin molecule in water. In the hemoglobin molecules of sickle-cell anemia patients, the —CH2CH2COOH chain is absent and in its place we find only the nonpolar (hydrophobic) —CH(CH3)2 group:
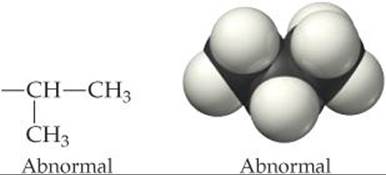
This change leads to the aggregation of the defective form of hemoglobin into particles too large to remain suspended in biological fluids. It also causes the cells to distort into the sickle shape shown in ![]() FIGURE 13.33. The sickled cells tend to clog capillaries, causing severe pain, weakness, and the gradual deterioration of vital organs. The disease is hereditary, and if both parents carry the defective genes, it is likely that their children will possess only abnormal hemoglobin.
FIGURE 13.33. The sickled cells tend to clog capillaries, causing severe pain, weakness, and the gradual deterioration of vital organs. The disease is hereditary, and if both parents carry the defective genes, it is likely that their children will possess only abnormal hemoglobin.
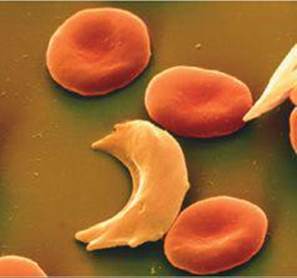
![]() FIGURE 13.33 Normal and sickled red blood cells. Normal red blood cells are about 1 × 10–3 mm in diameter.
FIGURE 13.33 Normal and sickled red blood cells. Normal red blood cells are about 1 × 10–3 mm in diameter.
SAMPLE INTEGRATIVE EXERCISE Putting Concepts Together
A 0.100-L solution is made by dissolving 0.441 g of CaCl2(s) in water. (a) Calculate the osmotic pressure of this solution at 27 °C, assuming that it is completely dissociated into its component ions. (b) The measured osmotic pressure of this solution is 2.56 atm at 27 °C. Explain why it is less than the value calculated in (a), and calculate the van't Hoff factor, i, for the solute in this solution. (See the “A Closer Look” box on Colligative Properties of Electrolyte Solutions in Section 13.5.) (c) The enthalpy of solution for CaCl2 is ΔH = –81.3 kJ/mol. If the final temperature of the solution is 27 °C, what was its initial temperature? (Assume that the density of the solution is 1.00 g/mL, that its specific heat is 4.18 J/g-K, and that the solution loses no heat to its surroundings.)
SOLUTION
(a) The osmotic pressure is given by Equation 13.14, Π = MRT. We know the temperature, T = 27 °C = 300 K, and the gas constant, R = 0.0821 L-atm/mol-K. We can calculate the molarity of the solution from the mass of CaCl2 and the volume of the solution:
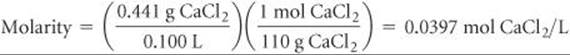
Soluble ionic compounds are strong electrolytes. ![]() (Sections 4.1 and 4.3) Thus, CaCl2 consists of metal cations (Ca2+) and nonmetal anions (Cl–). When completely dissociated, each CaCl2 unit forms three ions (one Ca2+ and two Cl–). Hence, the total concentration of ions in the solution is (3)(0.0397 M) = 0.119 M, and the calculated osmotic pressure is
(Sections 4.1 and 4.3) Thus, CaCl2 consists of metal cations (Ca2+) and nonmetal anions (Cl–). When completely dissociated, each CaCl2 unit forms three ions (one Ca2+ and two Cl–). Hence, the total concentration of ions in the solution is (3)(0.0397 M) = 0.119 M, and the calculated osmotic pressure is
Π = MRT = (0.119 mol/L)(0.0821 L-atm/mol-K)(300 K) = 2.93 atm
(b) The actual values of colligative properties of electrolytes are less than those calculated because the electrostatic interactions between ions limit their independent movements. In this case the van't Hoff factor, which measures the extent to which electrolytes actually dissociate into ions, is given by

Thus, the solution behaves as if the CaCl2 has dissociated into 2.62 particles instead of the ideal 3.
(c) If the solution is 0.0397 M in CaCl2 and has a total volume of 0.100 L, the number of moles of solute is (0.100 L)(0.0397 mol/L) = 0.00397 mol. Hence, the quantity of heat generated in forming the solution is (0.00397 mol)(–81.3 kJ/mol) = –0.323 kJ. The solution absorbs this heat, causing its temperature to increase. The relationship between temperature change and heat is given by Equation 5.22:
q = (specific heat)(grams)(ΔT)
The heat absorbed by the solution is q = +0.323 kJ = 323 J. The mass of the 0.100 L of solution is (100 mL)(1.00 g/mL) = 100 g (to three significant figures). Thus, the temperature change is

A kelvin has the same size as a degree Celsius. ![]() (Section 1.4) Because the solution temperature increases by 0.773 °C, the initial temperature was 27.0 °C – 0.773 °C = 26.2 °C.
(Section 1.4) Because the solution temperature increases by 0.773 °C, the initial temperature was 27.0 °C – 0.773 °C = 26.2 °C.