CHEMISTRY THE CENTRAL SCIENCE
14 CHEMICAL KINETICS
14.3 CONCENTRATION AND RATE LAWS
One way of studying the effect of concentration on reaction rate is to determine the way in which the initial rate of a reaction depends on the initial concentrations. For example, we might study the rate of the reaction
![]()
by measuring the concentration of NH4+ or NO2– as a function of time or by measuring the volume of N2 collected as a function of time. Because the stoichiometric coefficients on NH4+, NO2–, and N2 are the same, all of these rates are the same.
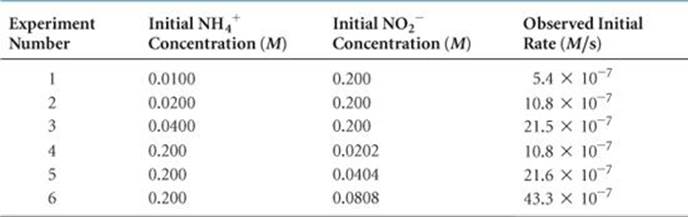
![]() TABLE 14.2 shows that changing the initial concentration of either reactant changes the initial reaction rate. If we double [NH4+] while holding [NO2–] constant, the rate doubles (compare experiments 1 and 2). If we increase [NH4+] by a factor of 4 but leave [NO2–] unchanged (experiments 1 and 3), the rate changes by a factor of 4, and so forth. These results indicate that the initial reaction rate is proportional to [NH4+]. When [NO2–] is similarly varied while [NH4+] is held constant, the rate is affected in the same manner. Thus, the rate is also directly proportional to the concentration of NO2–.
TABLE 14.2 shows that changing the initial concentration of either reactant changes the initial reaction rate. If we double [NH4+] while holding [NO2–] constant, the rate doubles (compare experiments 1 and 2). If we increase [NH4+] by a factor of 4 but leave [NO2–] unchanged (experiments 1 and 3), the rate changes by a factor of 4, and so forth. These results indicate that the initial reaction rate is proportional to [NH4+]. When [NO2–] is similarly varied while [NH4+] is held constant, the rate is affected in the same manner. Thus, the rate is also directly proportional to the concentration of NO2–.
TABLE 14.2 • Rate Data for the Reaction of Ammonium and Nitrite Ions in Water at 25 °C
 A CLOSER LOOK
A CLOSER LOOK
USING SPECTROSCOPIC METHODS TO MEASURE REACTION RATES
A variety of techniques can be used to monitor reactant and product concentration during a reaction, including spectroscopic methods, which rely on the ability of substances to absorb (or emit) light. Spectroscopic kinetic studies are often performed with the reaction mixture in the sample compartment of a spectrometer, an instrument that measures the amount of light transmitted or absorbed by a sample at different wavelengths. For kinetic studies, the spectrometer is set to measure the light absorbed at a wavelength characteristic of one of the reactants or products. In the decomposition of HI(g) into H2(g) and I2(g), for example, both HI and H2 are colorless, whereas I2 is violet. During the reaction, the violet color of the reaction mixture gets deeper as I2 forms. Thus, visible light of appropriate wavelength can be used to monitor the reaction (![]() FIGURE 14.5).
FIGURE 14.5).
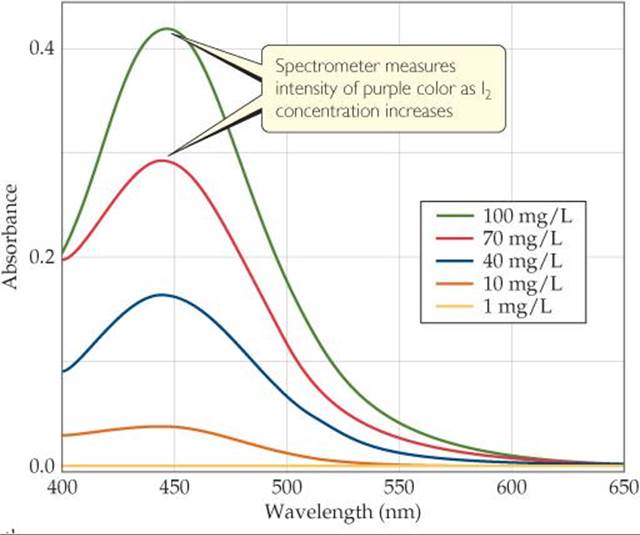
![]() FIGURE 14.5 Visible spectra of I2 at different concentrations.
FIGURE 14.5 Visible spectra of I2 at different concentrations.
![]() FIGURE 14.6 shows the components of a spectrometer. The spectrometer measures the amount of light absorbed by the sample by comparing the intensity of the light emitted from the light source with the intensity of the light transmitted through the sample, for various wavelengths. As the concentration of I2 increases and its color becomes more intense, the amount of light absorbed by the reaction mixture increases, as Figure 14.5 shows, causing less light to reach the detector.
FIGURE 14.6 shows the components of a spectrometer. The spectrometer measures the amount of light absorbed by the sample by comparing the intensity of the light emitted from the light source with the intensity of the light transmitted through the sample, for various wavelengths. As the concentration of I2 increases and its color becomes more intense, the amount of light absorbed by the reaction mixture increases, as Figure 14.5 shows, causing less light to reach the detector.
Beer's law relates the amount of light absorbed to the concentration of the absorbing substance:
![]()
In this equation, A is the measured absorbance, e is the extinction coefficient (a characteristic of the substance being monitored at a given wavelength of light), b is the path length through which the light passes, and c is the molar concentration of the absorbing substance. Thus, the concentration is directly proportional to absorbance. Many chemical and pharmaceutical companies routinely use Beer's law to calculate the concentration of purified solutions of the compounds that they make.
RELATED EXERCISES: 14.107, 14.108
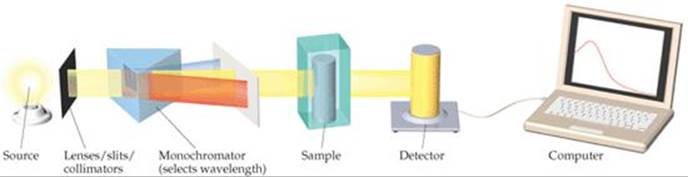
![]() FIGURE 14.6 Components of a spectrometer.
FIGURE 14.6 Components of a spectrometer.
We express the way in which the rate depends on the reactant concentrations by the equation
![]()
An equation such as Equation 14.6, which shows how the rate depends on reactant concentrations, is called a rate law. For the general reaction
![]()
the rate law generally has the form
![]()
The constant k is called the rate constant. The magnitude of k changes with temperature and therefore determines how temperature affects rate, as we will see in Section 14.5. The exponents m and n are typically small whole numbers. As we will learn shortly, if we know m and n for a reaction, we can gain great insight into the individual steps that occur during the reaction.
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
How do reaction rate, rate constant, and rate law differ?
Once we know the rate law for a reaction and the reaction rate for a set of reactant concentrations, we can calculate the value of k. For example, using the values for experiment 1 in Table 14.2, we can substitute into Equation 14.6:
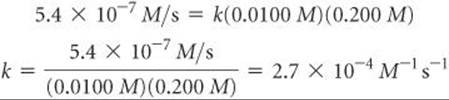
You may wish to verify that this same value of k is obtained using any of the other experimental results in Table 14.2.
Once we have both the rate law and the k value for a reaction, we can calculate the reaction rate for any set of concentrations. For example, using Equation 14.7 with k = 2.7 × 10–4M–1 s–1, m = 1, and n = 1, we can calculate the rate for [NH4+] = 0.100 M and [NO2–] = 0.100 M:
Rate = (2.7 × 10–4M–1 s–1)(0.100 M) (0.100 M) = 2.7 × 10–6M/s
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
Does the rate constant have the same units as the rate? Explain your answer.
Reaction Orders: The Exponents in the Rate Law
The rate law for most reactions has the form
![]()
The exponents m and n are called reaction orders. For example, consider again the rate law for the reaction of NH4+ with NO2–:
![]()
Because the exponent of [NH4+] is 1, the rate is first order in NH4+. The rate is also first order in NO2–. (The exponent 1 is not shown in rate laws.) The overall reaction order is the sum of the orders with respect to each reactant represented in the rate law. Thus, for the NH4+ – NO2–reaction, the rate law has an overall reaction order of 1 + 1 = 2, and the reaction is second order overall.
The exponents in a rate law indicate how the rate is affected by each reactant concentration. Because the rate at which NH4+ reacts with NO2– depends on [NH4+] raised to the first power, the rate doubles when [NH4+] doubles, triples when [NH4+] triples, and so forth. Doubling or tripling [NO2–] likewise doubles or triples the rate. If a rate law is second order with respect to a reactant, [A]2, then doubling the concentration of that substance causes the reaction rate to quadruple because [2]2 = 4, whereas tripling the concentration causes the rate to increase ninefold: [3]2= 9.
The following are some additional examples of experimentally determined rate laws:
![]()
![]()
![]()
Although the exponents in a rate law are sometimes the same as the coefficients in the balanced equation, this is not necessarily the case, as Equations 14.9 and 14.10 show. For any reaction, the rate law must be determined experimentally. In most rate laws, reaction orders are 0, 1, or 2. However, we also occasionally encounter rate laws in which the reaction order is fractional (such as Equation 14.10) or even negative.
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
The experimentally determined rate law for the reaction 2 NO(g) + 2 H2(g) → N2(g) + 2 H2O(g) is rate = k[NO]2[H2].
a. What are the reaction orders in this rate law?
b. Does doubling the concentration of NO have the same effect on rate as doubling the concentration of H2?
SAMPLE EXERCISE 14.4 Relating a Rate Law to the Effect of Concentration on Rate
Consider a reaction A + B → C for which rate = k[A][B]2. Each of the following boxes represents a reaction mixture in which A is shown as red spheres and B as purple ones. Rank these mixtures in order of increasing rate of reaction.
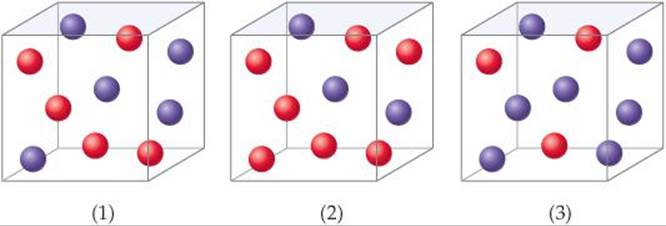
SOLUTION
Analyze We are given three boxes containing different numbers of spheres representing mixtures containing different reactant concentrations. We are asked to use the given rate law and the compositions of the boxes to rank the mixtures in order of increasing reaction rates.
Plan Because all three boxes have the same volume, we can put the number of spheres of each kind into the rate law and calculate the rate for each box.
Solve
Box 1 contains 5 red spheres and 5 purple spheres, giving the following rate:
Box 1: Rate = k(5)(5)2 = 125k
Box 2 contains 7 red spheres and 3 purple spheres:
Box 2: Rate = k(7)(3)2 = 63k
Box 3 contains 3 red spheres and 7 purple spheres:
Box 3: Rate = k(3)(7)2 = 147k
The slowest rate is 63k (box 2), and the highest is 147k (box 3). Thus, the rates vary in the order 2 < 1 < 3.
Check Each box contains 10 spheres. The rate law indicates that in this case [B] has a greater influence on rate than [A] because B has a higher reaction order. Hence, the mixture with the highest concentration of B (most purple spheres) should react fastest. This analysis confirms the order 2 < 1 < 3.
PRACTICE EXERCISE
Assuming that rate = k[A][B], rank the mixtures represented in this Sample Exercise in order of increasing rate.
Answer: 2 = 3 < 1
Magnitudes and Units of Rate Constants
If chemists want to compare reactions to evaluate which ones are relatively fast and which ones are relatively slow, the quantity of interest is the rate constant. A good general rule is that a large value of k (~109 or higher) means a fast reaction and a small value of k (10 or lower) means a slow reaction.
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
What does it mean if k is 0?
The units of the rate constant depend on the overall reaction order of the rate law. In a reaction that is second order overall, for example, the units of the rate constant must satisfy the equation:
Units of rate = (units of rate constant)(units of concentration)2
Hence, in our usual units of molarity for concentration and seconds for time, we have

SAMPLE EXERCISE 14.5 Determining Reaction Orders and Units for Rate Constants
(a) What are the overall reaction orders for the reactions described in Equations 14.9 and 14.10? (b) What are the units of the rate constant for the rate law in Equation 14.9?
SOLUTION
Analyze We are given two rate laws and asked to express (a) the overall reaction order for each and (b) the units for the rate constant for the first reaction.
Plan The overall reaction order is the sum of the exponents in the rate law. The units for the rate constant, k, are found by using the normal units for rate (M/s) and concentration (M) in the rate law and applying algebra to solve for k.
Solve
(a) The rate of the reaction in Equation 14.9 is first order in N2O5 and first order overall. The reaction in Equation 14.10 is first order in CHCl3 and one-half order in Cl2. The overall reaction order is three halves.
(b) For the rate law for Equation 14.9, we have
Units of rate = (units of rate constant)(units of concentration)
so
![]()
Notice that the units of the rate constant change as the overall order of the reaction changes.
PRACTICE EXERCISE
(a) What is the reaction order of the reactant H2 in Equation 14.11? (b) What are the units of the rate constant for Equation 14.11?
Answer: (a) 1, (b) M–1 s–1
Using Initial Rates to Determine Rate Laws
We have seen that the rate law for most reactions has the general form
Rate = k[reactant 1]m[reactant 2]n...
Thus, the task of determining the rate law becomes one of determining the reaction orders, m and n. In most reactions the reaction orders are 0, 1, or 2. If a reaction is zero order in a particular reactant, changing the concentration of that reactant has no effect on rate (as long as some of the reactant is present) because any concentration raised to the zero power equals 1. On the other hand, when a reaction is first order in a reactant, changes in the concentration of that reactant produce proportional changes in the rate. Thus, doubling the concentration will double the rate, and so forth. Finally, when the rate law is second order in a particular reactant, doubling its concentration increases the rate by a factor of 22 = 4, tripling its concentration causes the rate to increase by a factor of 32 = 9, and so forth.
In working with rate laws, it is important to realize that the rate of a reaction depends on concentration but the rate constant does not. As we will see later in this chapter, the rate constant (and hence the reaction rate) are affected by temperature and by the presence of a catalyst.
SAMPLE EXERCISE 14.6 Determining a Rate Law from Initial Rate Data
The initial rate of a reaction A + B → C was measured for several different starting concentrations of A and B, and the results are as follows:
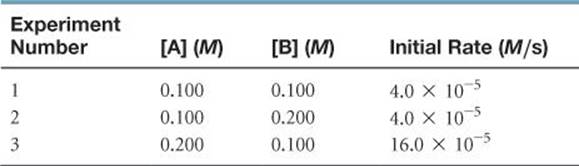
Using these data, determine (a) the rate law for the reaction, (b) the rate constant, (c) the rate of the reaction when [A] = 0.050 M and [B] = 0.100 M.
SOLUTION
Analyze We are given a table of data that relates concentrations of reactants with initial rates of reaction and asked to determine (a) the rate law, (b) the rate constant, and (c) the rate of reaction for a set of concentrations not listed in the table.
Plan
(a) We assume that the rate law has the following form: Rate = k[A]m[B]n. So we must use the given data to deduce the reaction orders m and n by determining how changes in the concentration change the rate. (b) Once we know m and n, we can use the rate law and one of the sets of data to determine the rate constant k. (c) Now that we know both the rate constant and the reaction orders, we can use the rate law with the given concentrations to calculate rate.
Solve
(a) If we compare experiments 1 and 2, we see that [A] is held constant and [B] is doubled. Thus, this pair of experiments shows how [B] affects the rate, allowing us to deduce the order of the rate law with respect to B. Because the rate remains the same when [B] is doubled, the concentration of B has no effect on the reaction rate. The rate law is therefore zero order in B (that is, n = 0).
In experiments 1 and 3, [B] is held constant, so these data show how [A] affects rate. Holding [B] constant while doubling [A] increases the rate fourfold. This result indicates that rate is proportional to [A]2 (that is, the reaction is second order in A). Hence, the rate law is
Rate = k[A]2[B]0 = k[A]2
(b) Using the rate law and the data from experiment 1, we have
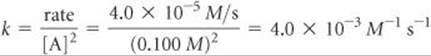
(c) Using the rate law from part (a) and the rate constant from part (b), we have
Rate = k[A]2 = (4.0 × 10–3M–1 s–1)(0.050 M)2 = 1.0 × 10–5M/s
Because [B] is not part of the rate law, it is irrelevant to the rate if there is at least some B present to react with A.
Check A good way to check our rate law is to use the concentrations in experiment 2 or 3 and see if we can correctly calculate the rate. Using data from experiment 3, we have
Rate = k[A]2 = (4.0 × 10–3M–1 s–1)(0.200 M)2 = 1.6 × 10–4M/s
Thus, the rate law correctly reproduces the data, giving both the correct number and the correct units for the rate.
PRACTICE EXERCISE
The following data were measured for the reaction of nitric oxide with hydrogen:
2 NO(g) + 2 H2(g) → N2(g) + 2 H2O(g)
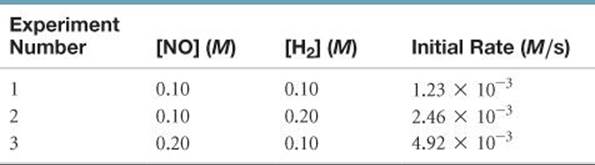
(a) Determine the rate law for this reaction. (b) Calculate the rate constant. (c) Calculate the rate when [NO] = 0.050 M and [H2] = 0.150 M.
Answer: (a) rate = k[NO]2[H2], (b) k = 1.2 M–2 s–1(c) rate = 4.5 × 10–4M/s