CHEMISTRY THE CENTRAL SCIENCE
15 CHEMICAL EQUILIBRIUM
15.3 UNDERSTANDING AND WORKING WITH EQUILIBRIUM CONSTANTS
Before doing calculations with equilibrium constants, it is valuable to understand what the magnitude of an equilibrium constant can tell us about the relative concentrations of reactants and products in an equilibrium mixture. It is also useful to consider how the magnitude of any equilibrium constant depends on how the chemical equation is expressed.
The Magnitude of Equilibrium Constants
The magnitude of the equilibrium constant for a reaction gives us important information about the composition of the equilibrium mixture. For example, consider the experimental data for the reaction of carbon monoxide gas and chlorine gas at 100 °C to form phosgene (COCl2), a toxic gas used in the manufacture of certain polymers and insecticides:
![]()
For the equilibrium constant to be so large, the numerator of the equilibrium-constant expression must be approximately a billion (109) times larger than the denominator. Thus, the equilibrium concentration of COCl2 must be much greater than that of CO or Cl2, and in fact this is just what we find experimentally. We say that this equilibrium lies to the right (that is, toward the product side). Likewise, a very small equilibrium constant indicates that the equilibrium mixture contains mostly reactants. We then say that the equilibrium lies to the left. In general,
If K ![]() 1 (large K): Equilibrium lies to right, products predominate
1 (large K): Equilibrium lies to right, products predominate
If K ![]() 1 (small K): Equilibrium lies to left, reactants predominate
1 (small K): Equilibrium lies to left, reactants predominate
These situations are summarized in ![]() FIGURE 15.6. Remember, it is forward and reverse rates that are equal at equilibrium, not concentrations.
FIGURE 15.6. Remember, it is forward and reverse rates that are equal at equilibrium, not concentrations.
![]() GO FIGURE
GO FIGURE
What would this figure look like for a reaction in which K ≈ 1?
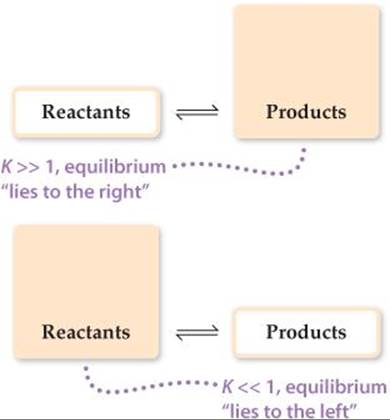
![]() FIGURE 15.6 Relationship between magnitude of K and composition of an equilibrium mixture.
FIGURE 15.6 Relationship between magnitude of K and composition of an equilibrium mixture.
SAMPLE EXERCISE 15.3 Interpreting the Magnitude of an Equilibrium Constant
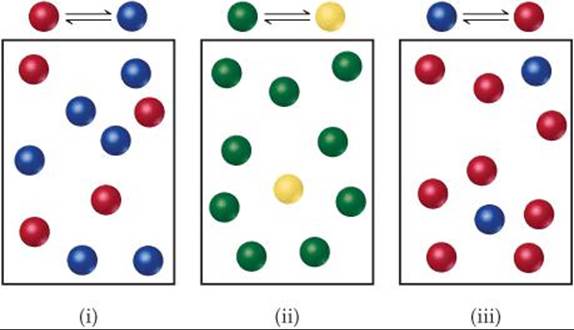
The following diagrams represent three systems at equilibrium, all in the same-size containers. (a) Without doing any calculations, rank the systems in order of increasing Kc. (b) If the volume of the containers is 1.0 L and each sphere represents 0.10 mol, calculate Kc for each system.
SOLUTION
Analyze We are asked to judge the relative magnitudes of three equilibrium constants and then to calculate them.
Plan (a) The more product present at equilibrium, relative to reactant, the larger the equilibrium constant. (b) The equilibrium constant is given by Equation 15.8.
Solve
(a) Each box contains 10 spheres. The amount of product in each varies as follows: (i) 6, (ii) 1, (iii) 8. Therefore, the equilibrium constant varies in the order (ii) < (i) < (iii), from smallest (most reactant) to largest (most products).
(b) In (i) we have 0.60 mol/L product and 0.40 mol/L reactant, giving Kc = 0.60/0.40 = 1.5. (You will get the same result by merely dividing the number of spheres of each kind: 6 spheres/4 spheres = 1.5.) In (ii) we have 0.10 mol/L product and 0.90 mol/L reactant, giving Kc = 0.10/0.90 = 0.11 (or 1 sphere/9 spheres = 0.11). In (iii) we have 0.80 mol/L product and 0.20 mol/L reactant, giving Kc = 0.80/0.20 = 4.0 (or 8 spheres/2 spheres = 4.0). These calculations verify the order in (a).
Comment Imagine a drawing that represents a reaction with a very small or very large value of Kc. For example, what would the drawing look like if Kc = 1 × 10–5 In that case there would need to be 100,000 reactant molecules for only 1 product molecule. But then, that would be impractical to draw.
PRACTICE EXERCISE
For the reaction ![]() at 298 K and Kp = 55 at 700 K. Is the formation of HI favored more at the higher or lower temperature?
at 298 K and Kp = 55 at 700 K. Is the formation of HI favored more at the higher or lower temperature?
Answer: at the lower temperature because Kp is larger at the lower temperature
The Direction of the Chemical Equation and K
We have seen that we can represent the N2O4/NO2 equilibrium as
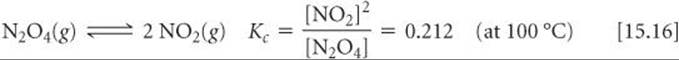
We could equally well consider this equilibrium in terms of the reverse reaction:
![]()
The equilibrium expression is then

Equation 15.17 is the reciprocal of the expression in Equation 15.16. The equilibrium-constant expression for a reaction written in one direction is the reciprocal of the expression for the reaction written in the reverse direction. Consequently, the numerical value of the equilibrium constant for the reaction written in one direction is the reciprocal of that for the reverse reaction. Both expressions are equally valid, but it is meaningless to say that the equilibrium constant for the equilibrium between NO2 and N2O4 is “0.212” or “4.72” unless we indicate how the equilibrium reaction is written and specify the temperature. Therefore, whenever you are using an equilibrium constant, you should always write the associated balanced chemical equation.
SAMPLE EXERCISE 15.4 Evaluating an Equilibrium Constant When an Equation Is Reversed
For the reaction
![]()
SOLUTION
Analyze We are asked to write the equilibrium-constant expression for a reaction and to determine the value of Kc given the chemical equation and equilibrium constant for the reverse reaction. that is run at 25 °C, Kc = 1 × 10–30. Use this information to write the equilibrium-constant expression and calculate the equilibrium constant for the reaction
![]()
Plan The equilibrium-constant expression is a quotient of products over reactants, each raised to a power equal to its coefficient in the balanced equation. The value of the equilibrium constant is the reciprocal of that for the reverse reaction.
Solve
Writing products over reactants, we have
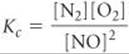
Both the equilibrium-constant expression and the numerical value of the equilibrium constant are the reciprocals of those for the formation of NO from N2 and O2:

Comment Regardless of the way we express the equilibrium among NO, N2, and O2, at 25 °C it lies on the side that favors N2 and O2. Thus, the equilibrium mixture will contain mostly N2 and O2 with very little NO present.
PRACTICE EXERCISE
For ![]() What is the value of Kp for the reverse reaction?
What is the value of Kp for the reverse reaction?
Answer: 2.30 × 102
Relating Chemical Equation Stoichiometry and Equilibrium Constants
There are many ways to write a balanced chemical equation for a given reaction. For example, if we multiply Equation 15.1, ![]() by 2, we have
by 2, we have
![]()
This chemical equation is balanced and might be written this way in some contexts. Therefore, the equilibrium-constant expression for this equation is
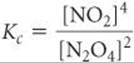
which is the square of the equilibrium-constant expression given in Equation 15.10 for the reaction as written in Equation 15.1: [NO2]2/[N2O4]. Because the new equilibrium-constant expression equals the original expression squared, the new equilibrium constant Kc equals the original constant squared: 0.2122 = 0.0449 (at 100 °C). Once again, it is important to remember that you must relate each equilibrium constant you work with to a specific balanced chemical equation. The concentrations of the substances in the equilibrium mixture will be the same no matter how you write the chemical equation, but the value of Kc you calculate depends completely on how you write the reaction.
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
How does the magnitude of Kp for the reaction ![]() change if the equilibrium is written 6
change if the equilibrium is written 6 ![]()
It is also possible to calculate the equilibrium constant for a reaction if we know the equilibrium constants for other reactions that add up to give us the one we want, similar to Hess's law. ![]() (Section 5.6) For example, consider the following two reactions, their equilibrium-constant expressions, and their equilibrium constants at 100 °C:
(Section 5.6) For example, consider the following two reactions, their equilibrium-constant expressions, and their equilibrium constants at 100 °C:
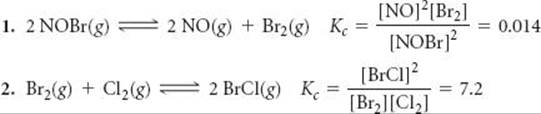
The net sum of these two equations is
![]()
You can prove algebraically that the equilibrium-constant expression for reaction 3 is the product of the expressions for reactions 1 and 2:
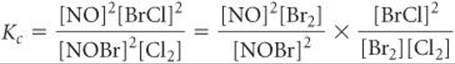
Thus,
![]()
To summarize:
1. The equilibrium constant of a reaction in the reverse direction is the inverse (or reciprocal) of the equilibrium constant of the reaction in the forward direction:
![]()
2. The equilibrium constant of a reaction that has been multiplied by a number is equal to the original equilibrium constant raised to a power equal to that number.
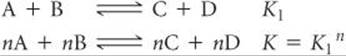
3. The equilibrium constant for a net reaction made up of two or more reactions is the product of the equilibrium constants for the individual reactions:
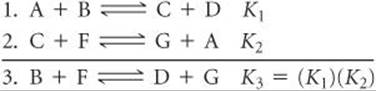
SAMPLE EXERCISE 15.5 Combining Equilibrium Expressions
Given the reactions
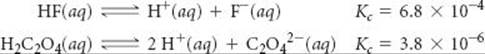
SOLUTION
Analyze We are given two equilibrium equations and the corresponding equilibrium constants and are asked to determine the equilibrium constant for a third equation, which is related to the first two. determine the value of Kc for the reaction
![]()
Plan We cannot simply add the first two equations to get the third. Instead, we need to determine how to manipulate the equations to come up with the steps that will add to give us the desired equation.
Solve
If we multiply the first equation by 2 and make the corresponding change to its equilibrium constant (raising to the power 2), we get
![]()
Reversing the second equation and again making the corresponding change to its equilibrium constant (taking the reciprocal) gives
![]()
Now we have two equations that sum to give the net equation, and we can multiply the individual Kc values to get the desired equilibrium constant.
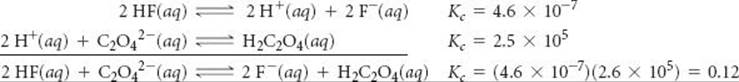
PRACTICE EXERCISE
Given that, at 700 K, Kp = 54.0 for the reaction ![]() for the reaction N2(g) + 3 H2(g)
for the reaction N2(g) + 3 H2(g) ![]() NH3(g) determine the value of Kp for the reaction
NH3(g) determine the value of Kp for the reaction ![]()
![]()
Answer: ![]()