CHEMISTRY THE CENTRAL SCIENCE
2 ATOMS, MOLECULES, AND IONS
2.6 MOLECULES AND MOLECULAR COMPOUNDS
Even though the atom is the smallest representative sample of an element, only the noble-gas elements are normally found in nature as isolated atoms. Most matter is composed of molecules or ions. We examine molecules here and ions in Section 2.7.
Molecules and Chemical Formulas
Several elements are found in nature in molecular form—two or more of the same type of atom bound together. For example, most of the oxygen in air consists of molecules that contain two oxygen atoms. As we saw in Section 1.2, we represent this molecular oxygen by the chemical formula O2 (read “oh two”). The subscript tells us that two oxygen atoms are present in each molecule. A molecule made up of two atoms is called a diatomic molecule.
Oxygen also exists in another molecular form known as ozone. Molecules of ozone consist of three oxygen atoms, making the chemical formula O3. Even though “normal” oxygen (O2) and ozone (O3) are both composed only of oxygen atoms, they exhibit very different chemical and physical properties. For example, O2 is essential for life, but O3 is toxic; O2 is odorless, whereas O3 has a sharp, pungent smell.
The elements that normally occur as diatomic molecules are hydrogen, oxygen, nitrogen, and the halogens (H2, O2, N2, F2, Cl2, Br2, and I2). Except for hydrogen, these diatomic elements are clustered on the right side of the periodic table.
Compounds composed of molecules contain more than one type of atom and are called molecular compounds. A molecule of the compound methane, for example, consists of one carbon atom and four hydrogen atoms and is therefore represented by the chemical formula CH4. Lack of a subscript on the C indicates one atom of C per methane molecule. Several common molecules of both elements and compounds are shown in ![]() FIGURE 2.18. Notice how the composition of each substance is given by its chemical formula. Notice also that these substances are composed only of nonmetallic elements. Most molecular substances we will encounter contain only nonmetals.
FIGURE 2.18. Notice how the composition of each substance is given by its chemical formula. Notice also that these substances are composed only of nonmetallic elements. Most molecular substances we will encounter contain only nonmetals.
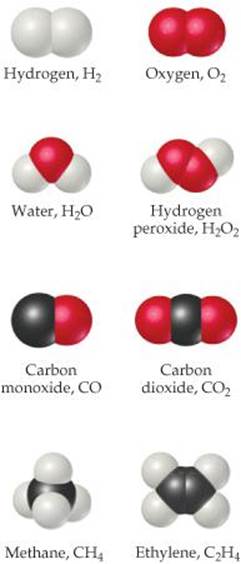
![]() Figure 2.18 Molecular models. Notice how the chemical formulas of these simple molecules correspond to their compositions.
Figure 2.18 Molecular models. Notice how the chemical formulas of these simple molecules correspond to their compositions.
Molecular and Empirical Formulas
Chemical formulas that indicate the actual numbers of atoms in a molecule are called molecular formulas. (The formulas in Figure 2.18 are molecular formulas.) Chemical formulas that give only the relative number of atoms of each type in a molecule are called empirical formulas. The subscripts in an empirical formula are always the smallest possible whole-number ratios. The molecular formula for hydrogen peroxide is H2O2, for example, whereas its empirical formula is HO. The molecular formula for ethylene is C2H4, and its empirical formula is CH2. For many substances, the molecular formula and the empirical formula are identical, as in the case of water, H2O.
Whenever we know the molecular formula of a compound, we can determine its empirical formula. The converse is not true, however. If we know the empirical formula of a substance, we cannot determine its molecular formula unless we have more information. So why do chemists bother with empirical formulas? As we will see in Chapter 3, certain common methods of analyzing substances lead to the empirical formula only. Once the empirical formula is known, additional experiments can give the information needed to convert the empirical formula to the molecular one. In addition, there are substances that do not exist as isolated molecules. For these substances, we must rely on empirical formulas.
SAMPLE EXERCISE 2.6 Relating Empirical and Molecular Formulas
Write the empirical formulas for (a) glucose, a substance also known as either blood sugar or dextrose, molecular formula C6H12O6; (b) nitrous oxide, a substance used as an anesthetic and commonly called laughing gas, molecular formula N2O.
SOLUTION
(a) The subscripts of an empirical formula are the smallest whole-number ratios. The smallest ratios are obtained by dividing each subscript by the largest common factor, in this case 6. The resultant empirical formula for glucose is CH2O.
(b) Because the subscripts in N2O are already the lowest integral numbers, the empirical formula for nitrous oxide is the same as its molecular formula, N2O.
PRACTICE EXERCISE
Give the empirical formula for diborane, whose molecular formula is B2H6.
Answer: BH3
Picturing Molecules
The molecular formula of a substance summarizes the composition of the substance but does not show how the atoms are joined together in the molecule. A structural formula shows which atoms are attached to which, as in the following examples:
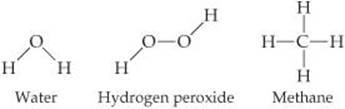
The atoms are represented by their chemical symbols, and lines are used to represent the bonds that hold the atoms together.
A structural formula usually does not depict the actual geometry of the molecule, that is, the actual angles at which atoms are joined together. A structural formula can be written as a perspective drawing (![]() FIGURE 2.19), however, to give some sense of three-dimensional shape.
FIGURE 2.19), however, to give some sense of three-dimensional shape.
Scientists also rely on various models to help visualize molecules. Ball-and-stick models show atoms as spheres and bonds as sticks. This type of model has the advantage of accurately representing the angles at which the atoms are attached to one another in the molecule (Figure 2.19). Sometimes the chemical symbols of the elements are superimposed on the balls, but often the atoms are identified simply by color.
A space-filling model depicts what the molecule would look like if the atoms were scaled up in size (Figure 2.19). These models show the relative sizes of the atoms, but the angles between atoms, which help define their molecular geometry, are often more difficult to see than in ball-and-stick models. As in ball-and-stick models, the identities of the atoms are indicated by color, but they may also be labeled with the element's symbol.
![]() GO FIGURE
GO FIGURE
What advantage does a ball-and-stick model have over a spacefilling model?
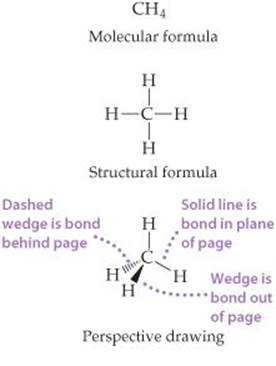
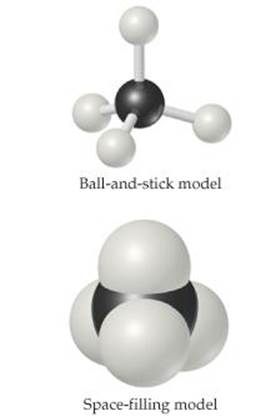
![]() Figure 2.19 Different representations of the methane (CH4) molecule. Structural formulas, perspective drawings, ball-and-stick models, and spacefilling models correspond to the molecular formula, and each helps us visualize the ways atoms are attached to each other.
Figure 2.19 Different representations of the methane (CH4) molecule. Structural formulas, perspective drawings, ball-and-stick models, and spacefilling models correspond to the molecular formula, and each helps us visualize the ways atoms are attached to each other.
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
The structural formula for ethane is
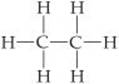
a. What is the molecular formula for ethane?
b. What is its empirical formula?
c. Which kind of molecular model would most clearly show the angles between atoms?