CHEMISTRY THE CENTRAL SCIENCE
16 ACID–BASE EQUILIBRIA

RHUBARB is a vegetable whose stalks are eaten for their tart flavor. The sour taste comes from the presence of acids in the plant.
WHAT'S AHEAD
16.1 ACIDS AND BASES: A BRIEF REVIEW
We start by reviewing the Arrhenius definition of acids and bases.
16.2 BRØNSTED–LOWRY ACIDS AND BASES
We learn that a Brønsted–Lowry acid is a proton donor and a Brønsted–Lowry base is a proton acceptor. Two species that differ by the presence or absence of a proton are known as a conjugate acid–base pair.
16.3 THE AUTOIONIZATION OF WATER
We see that the autoionization of water produces small quantities of H3O+ and OH– ions. The equilibrium constant for autoionization, Kw = [H3O+][OH–], defines the relationship between H3O+ and OH– concentrations in aqueous solutions.
16.4 THE pH SCALE
We use the pH scale to describe the acidity or basicity of an aqueous solution. Neutral solutions have a pH = 7, acidic solutions have pH below 7, and basic solutions have pH above 7.
16.5 STRONG ACIDS AND BASES
We categorize acids and bases as being either strong or weak electrolytes. Strong acids and bases are strong electrolytes, ionizing or dissociating completely in aqueous solution. Weak acids and bases are weak electrolytes and ionize only partially.
16.6 WEAK ACIDS
We learn that the ionization of a weak acid in water is an equilibrium process with an equilibrium constant Ka that can be used to calculate the pH of a weak acid solution.
16.7 WEAK BASES
We learn that the ionization of a weak base in water is an equilibrium process with equilibrium constant Kb that can be used to calculate the pH of a weak base solution.
16.8 RELATIONSHIP BETWEEN Ka AND Kb
We see that the relationship Ka × Kb = Kw means that the stronger an acid, the weaker its conjugate base.
16.9 ACID–BASE PROPERTIES OF SALT SOLUTIONS
We explore the fact that the ions of a soluble ionic compound can serve as Brønsted–Lowry acids or bases.
16.10 ACID–BASE BEHAVIOR AND CHEMICAL STRUCTURE
We explore the relationship between chemical structure and acid–base behavior.
16.11 LEWIS ACIDS AND BASES
Finally, we learn that a Lewis acid is an electron-pair acceptor and a Lewis base is an electron-pair donor.
TASTE IS ONE OF THE five senses we use to experience the world around us. Receptors on the tongue are sensitive to chemical stimuli that lead to five basic taste sensations: sweet, sour, salty, bitter, and umami (from the Japanese word for “delicious” and triggered by the amino acid glutamic acid). The sensation of sour is a response to the presence of acids, and we associate a sour taste with certain fruits and vegetables because they contain acids. For example, lemons, limes, and grapefruits contain citric acid (H3C6H5O7), and green apples and grapes contain malic acid (H2C4H4O5). The vegetable rhubarb is among the sourest of foods, so sour that eating a fresh stalk of rhubarb is sure to elicit a pucker on the first bite. The sour taste comes from the high acid content of the stalks. One of several acids found in rhubarb is oxalic acid, (H2C2O4) (![]() FIGURE 16.1), which in large doses can be lethal. The oxalic acid content in the leaves is much higher than in the stalks, so much so that the leaves are considered toxic.*
FIGURE 16.1), which in large doses can be lethal. The oxalic acid content in the leaves is much higher than in the stalks, so much so that the leaves are considered toxic.*
Acids and bases are important in numerous chemical processes that occur around us—from industrial processes to biological ones, from reactions in the laboratory to those in our environment. The time required for a metal object immersed in water to corrode, the ability of an aquatic environment to support fish and plant life, the fate of pollutants washed out of the air by rain, and even the rates of reactions that maintain our lives all critically depend on the acidity or basicity of solutions.
We have encountered acids and bases many times in earlier discussions. For example, a portion of Chapter 4 focused on their reactions. In this chapter we reexamine acids and bases, taking a closer look at how they are identified and characterized. In doing so, we consider their behavior both in terms of their structure and bonding and in terms of the chemical equilibria in which they participate.
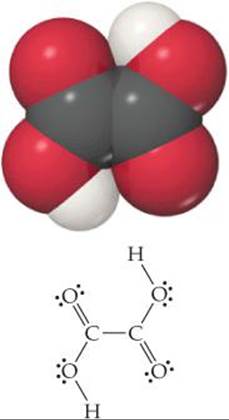
![]() FIGURE 16.1 Oxalic acid, H2C2O4.
FIGURE 16.1 Oxalic acid, H2C2O4.
16.1 ACIDS AND BASES: A BRIEF REVIEW
From the earliest days of experimental chemistry, scientists have recognized acids and bases by their characteristic properties. Acids have a sour taste and cause certain dyes to change color, whereas bases have a bitter taste and feel slippery (soap is a good example). Use of the term basecomes from the old English meaning of the word, “to bring low.” (We still use the word debase in this sense, meaning to lower the value of something.) When a base is added to an acid, the base “lowers” the amount of acid. Indeed, when acids and bases are mixed in certain proportions, their characteristic properties disappear altogether. ![]() (Section 4.3)
(Section 4.3)
By 1830 it was evident that all acids contain hydrogen but not all hydrogen-containing substances are acids. During the 1880s, the Swedish chemist Svante Arrhenius (1859–1927) defined acids as substances that produce H+ ions in water and bases as substances that produce OH– ions in water. Over time the Arrhenius concept of acids and bases came to be stated in the following way:
• An acid is a substance that, when dissolved in water, increases the concentration of H+ ions.
• A base is a substance that, when dissolved in water, increases the concentration of OH– ions.
Hydrogen chloride is an Arrhenius acid. Hydrogen chloride gas is highly soluble in water because of its chemical reaction with water, which produces hydrated H+ and Cl– ions:
![]()
The aqueous solution of HCl is known as hydrochloric acid. Concentrated hydrochloric acid is about 37% HCl by mass and is 12 M in HCl.
Sodium hydroxide is an Arrhenius base. Because NaOH is an ionic compound, it dissociates into Na+ and OH– ions when it dissolves in water, thereby releasing OH– ions into the solution.
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
What two ions are central to the Arrhenius definitions of acids and bases?