CHEMISTRY THE CENTRAL SCIENCE
16 ACID–BASE EQUILIBRIA
16.6 WEAK ACIDS
Most acidic substances are weak acids and therefore only partially ionized in aqueous solution (![]() FIGURE 16.9). We can use the equilibrium constant for the ionization reaction to express the extent to which a weak acid ionizes. If we represent a general weak acid as HA, we can write the equation for its ionization in either of the following ways, depending on whether the hydrated proton is represented as H3O+(aq) or H+(aq):
FIGURE 16.9). We can use the equilibrium constant for the ionization reaction to express the extent to which a weak acid ionizes. If we represent a general weak acid as HA, we can write the equation for its ionization in either of the following ways, depending on whether the hydrated proton is represented as H3O+(aq) or H+(aq):
![]()
or
![]()
Because H2O is the solvent, it is omitted from the equilibrium-constant expression. ![]() (Section 15.4) Thus, we can write the equilibrium-constant expression as either
(Section 15.4) Thus, we can write the equilibrium-constant expression as either
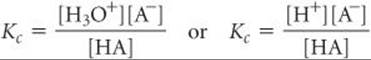
As we did for the ion-product constant for the autoionization of water, we change the subscript on this equilibrium constant to indicate the type of equation to which it corresponds:
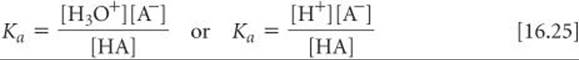
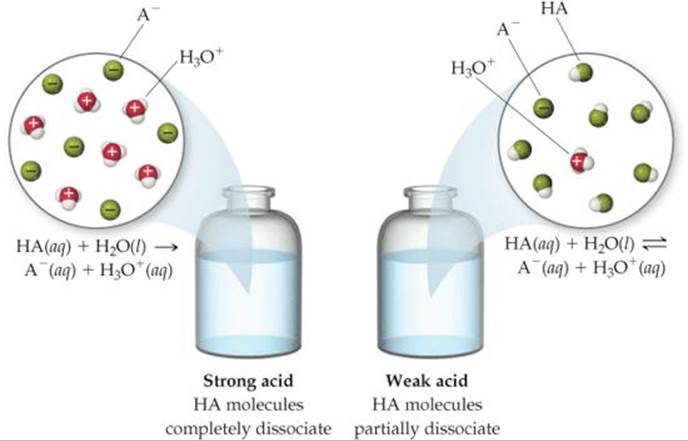
![]() FIGURE 16.9 Species present in a solution of a strong acid and a weak acid.
FIGURE 16.9 Species present in a solution of a strong acid and a weak acid.
The subscript a denotes that Ka is an equilibrium constant for the ionization of an acid, so Ka is called the acid-dissociation constant.
![]() TABLE 16.2 shows the structural formulas, conjugate bases, and Ka values for a number of weak acids. Appendix D provides a more complete list. Many weak acids are organic compounds composed entirely of carbon, hydrogen, and oxygen. These compounds usually contain some hydrogen atoms bonded to carbon atoms and some bonded to oxygen atoms. In almost all cases, the hydrogen atoms bonded to carbon do not ionize in water; instead, the acidic behavior of these compounds is due to the hydrogen atoms attached to oxygen atoms.
TABLE 16.2 shows the structural formulas, conjugate bases, and Ka values for a number of weak acids. Appendix D provides a more complete list. Many weak acids are organic compounds composed entirely of carbon, hydrogen, and oxygen. These compounds usually contain some hydrogen atoms bonded to carbon atoms and some bonded to oxygen atoms. In almost all cases, the hydrogen atoms bonded to carbon do not ionize in water; instead, the acidic behavior of these compounds is due to the hydrogen atoms attached to oxygen atoms.
TABLE 16.2 • Some Weak Acids in Water at 25 °C
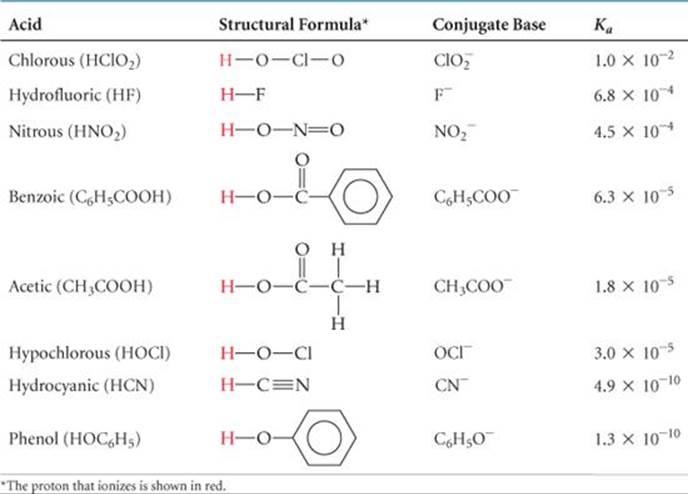
The magnitude of Ka indicates the tendency of the acid to ionize in water: The larger the value of Ka, the stronger the acid. Chlorous acid (HClO2), for example, is the strongest acid in Table 16.2, and phenol (HOC6H5) is the weakest. For most weak acids Ka values range from 10–2 to 10–10.
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
Based on the entries in Table 16.2, which element is most commonly bonded to the acidic hydrogen?
Calculating Ka from pH
In order to calculate either the Ka value for a weak acid or the pH of its solutions, we will use many of the skills for solving equilibrium problems developed in Section 15.5. In many cases the small magnitude of Ka allows us to use approximations to simplify the problem. In doing these calculations, it is important to realize that proton-transfer reactions are generally very rapid. As a result, the measured or calculated pH for a weak acid always represents an equilibrium condition.
SAMPLE EXERCISE 16.10 Calculating Ka from Measured pH
A student prepared a 0.10 M solution of formic acid (HCOOH) and found its pH at 25 °C to be 2.38. Calculate Ka for formic acid at this temperature.
SOLUTION
Analyze We are given the molar concentration of an aqueous solution of weak acid and the pH of the solution, and we are asked to determine the value of Ka for the acid.
Plan Although we are dealing specifically with the ionization of a weak acid, this problem is very similar to the equilibrium problems we encountered in Chapter 15. We can solve this problem using the method first outlined in Sample Exercise 15.9, starting with the chemical reaction and a tabulation of initial and equilibrium concentrations.
Solve The first step in solving any equilibrium problem is to write the equation for the equilibrium reaction. The ionization of formic acid can be written as
![]()
The equilibrium-constant expression is
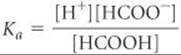
From the measured pH, we can calculate [H+]:
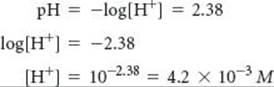
To determine the concentrations of the species involved in the equilibrium, we imagine that the solution is initially 0.10 M in HCOOH molecules. We then consider the ionization of the acid into H+ and HCOO–. For each HCOOH molecule that ionizes, one H+ ion and one HCOO– ion are produced in solution. Because the pH measurement indicates that [H+] = 4.2 × 10–3M at equilibrium, we can construct the following table:

Notice that we have neglected the very small concentration of H+(aq) due to H2O autoionization. Notice also that the amount of HCOOH that ionizes is very small compared with the initial concentration of the acid. To the number of significant figures we are using, the subtraction yields 0.10 M:
![]()
We can now insert the equilibrium concentrations into the expression for Ka:

Check The magnitude of our answer is reasonable because Ka for a weak acid is usually between 10–2 and 10–10.
PRACTICE EXERCISE
Niacin, one of the B vitamins, has the molecular structure
A 0.020 M solution of niacin has a pH of 3.26. What is the acid-dissociation constant for niacin?
Answer: 1.5 × 10–5
Percent Ionization
We have seen that the magnitude of Ka indicates the strength of a weak acid. Another measure of acid strength is percent ionization, defined as

The stronger the acid, the greater the percent ionization.
For any acid, the concentration of acid that ionizes equals the concentration of H+(aq) that forms, assuming that H2O autoionization is negligible. Thus, the percent ionization for an acid HA is also given by

For example, a 0.035 M solution of HNO2 contains 3.7 × 10–3M H+(aq) and its percent ionization is
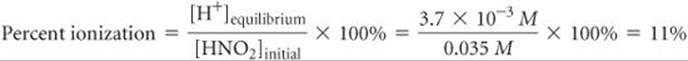
SAMPLE EXERCISE 16.11 Calculating Percent Ionization
As calculated in Sample Exercise 16.10, a 0.10 M solution of formic acid (HCOOH) contains 4.2 × 10–3M H+(aq). Calculate the percentage of the acid that is ionized.
SOLUTION
Analyze We are given the molar concentration of an aqueous solution of weak acid and the equilibrium concentration of H+(aq) and asked to determine the percent ionization of the acid.
Plan The percent ionization is given by Equation 16.27.
Solve
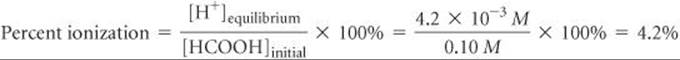
PRACTICE EXERCISE
A 0.020 M solution of niacin has a pH of 3.26. Calculate the percent ionization of the niacin.
Answer: 2.7%
Using Ka to Calculate pH
Knowing the value of Ka and the initial concentration of a weak acid, we can calculate the concentration of H+(aq) in a solution of the acid. Let's calculate the pH at 25 °C of a 0.30 M solution of acetic acid (CH3COOH), the weak acid responsible for the characteristic odor and acidity of vinegar.
1. Our first step is to write the ionization equilibrium:
![]()
Notice that the hydrogen that ionizes is the one attached to an oxygen atom.
2. The second step is to write the equilibrium-constant expression and the value for the equilibrium constant. Taking Ka = 1.8 × 10–5 from Table 16.2, we write
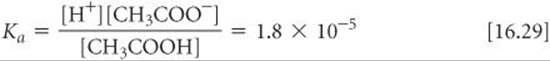
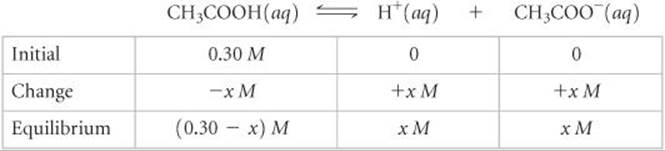
3. The third step is to express the concentrations involved in the equilibrium reaction. This can be done with a little accounting, as described in Sample Exercise 16.10. Because we want to find the equilibrium value for [H+], let's call this quantity x. The concentration of acetic acid before any of it ionizes is 0.30 M. The chemical equation tells us that for each molecule of CH3COOH that ionizes, one H+(aq) and one CH3COO–(aq) are formed. Consequently, if x moles per liter of H+(aq) form at equilibrium, x moles per liter of CH3COO–(aq) must also form andx moles per liter of CH3COOH must be ionized:
4. The fourth step is to substitute the equilibrium concentrations into the equilibrium-constant expression and solve for x:
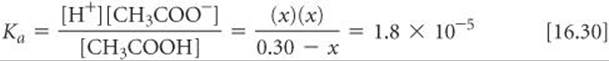
This expression leads to a quadratic equation in x, which we can solve by using either an equation-solving calculator or the quadratic formula. We can simplify the problem, however, by noting that the value of Ka is quite small. As a result, we anticipate that the equilibrium lies far to the left and that x is much smaller than the initial concentration of acetic acid. Thus, we assume that x is negligible relative to 0.30, so that 0.30 – x is essentially equal to 0.30. We can (and should!) check the validity of this assumption when we finish the problem. By using this assumption, Equation 16.30 becomes
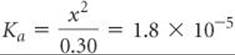
Solving for x, we have
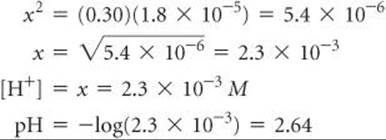
Now we check the validity of our simplifying assumption that ![]() . The value of x we determined is so small that, for this number of significant figures, the assumption is entirely valid. We are thus satisfied that the assumption was a reasonable one to make. Because xrepresents the moles per liter of acetic acid that ionize, we see that, in this particular case, less than 1% of the acetic acid molecules ionize:
. The value of x we determined is so small that, for this number of significant figures, the assumption is entirely valid. We are thus satisfied that the assumption was a reasonable one to make. Because xrepresents the moles per liter of acetic acid that ionize, we see that, in this particular case, less than 1% of the acetic acid molecules ionize:
As a general rule, if x is more than about 5% of the initial concentration value, it is better to use the quadratic formula. You should always check the validity of any simplifying assumptions after you have finished solving a problem.
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
Why can we generally assume that the equilibrium concentration of a weak acid equals its initial concentration?
Finally, we can compare the pH value of this weak acid with the pH of a solution of a strong acid of the same concentration. The pH of the 0.30 M acetic acid is 2.64, but the pH of a 0.30 M solution of a strong acid such as HCl is –log(0.30) = 0.52. As expected, the pH of a solution of a weak acid is higher than that of a solution of a strong acid of the same molarity. (Remember, the higher the pH value, the less acidic the solution.)
SAMPLE EXERCISE 16.12 Using Ka to Calculate pH
Calculate the pH of a 0.20 M solution of HCN. (Refer to Table 16.2 or Appendix D for the value of Ka.)
SOLUTION
Analyze We are given the molarity of a weak acid and are asked for the pH. From Table 16.2, Ka for HCN is 4.9 × 10–10.
Plan We proceed as in the example just worked in the text, writing the chemical equation and constructing a table of initial and equilibrium concentrations in which the equilibrium concentration of H+ is our unknown.
Solve Writing both the chemical equation for the ionization reaction that forms H+(aq) and the equilibirium-constant (Ka) expression for the reaction:
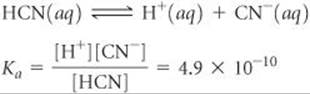
Next, we tabulate the concentrations of the species involved in the equilibrium reaction, letting x = [H+] at equilibrium:

Substituting the equilibrium concentrations into the equilibrium-constant expression yields
![]()
We next make the simplifying approximation that x, the amount of acid that dissociates, is small compared with the initial concentration of acid, ![]() . Thus,
. Thus,
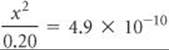
Solving for x, we have
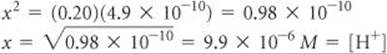
A concentration of 9.9 × 10–6M is much smaller than 5% of 0.20, the initial HCN concentration. Our simplifying approximation is therefore appropriate. We now calculate the pH of the solution:
![]()
PRACTICE EXERCISE
The Ka for niacin (Practice Exercise 16.10) is 1.5 × 10–5. What is the pH of a 0.010 M solution of niacin?
Answer: 3.41

![]() FIGURE 16.10 Rates of the same reaction run in a weak acid and a strong acid. The bubbles are H2 gas, which along with metal cations, is produced when a metal is oxidized by an acid (Section 4.4).
FIGURE 16.10 Rates of the same reaction run in a weak acid and a strong acid. The bubbles are H2 gas, which along with metal cations, is produced when a metal is oxidized by an acid (Section 4.4).
The properties of an acid solution that relate directly to the concentration of H+(aq), such as electrical conductivity and rate of reaction with an active metal, are less evident for a solution of a weak acid than for a solution of a strong acid of the same concentration. ![]() FIGURE 16.10presents an experiment that demonstrates this difference with 1 M CH3COOH and 1 M HCl. The 1 M CH3COOH contains only 0.004 M H+(aq), whereas the 1 M HCl solution contains 1 M H+(aq). As a result, the reaction rate with the metal is much faster in the HCl solution.
FIGURE 16.10presents an experiment that demonstrates this difference with 1 M CH3COOH and 1 M HCl. The 1 M CH3COOH contains only 0.004 M H+(aq), whereas the 1 M HCl solution contains 1 M H+(aq). As a result, the reaction rate with the metal is much faster in the HCl solution.
As the concentration of a weak acid increases, the equilibrium concentration of H+(aq) increases, as expected. However, as shown in ![]() FIGURE 16.11, the percent ionization decreases as the concentration increases. Thus, the concentration of H+(aq) is not directly proportional to the concentration of the weak acid. For example, doubling the concentration of a weak acid does not double the concentration of H+(aq).
FIGURE 16.11, the percent ionization decreases as the concentration increases. Thus, the concentration of H+(aq) is not directly proportional to the concentration of the weak acid. For example, doubling the concentration of a weak acid does not double the concentration of H+(aq).
![]() GO FIGURE
GO FIGURE
Is the trend observed in this graph consistent with Le Châtelier's principle? Explain.
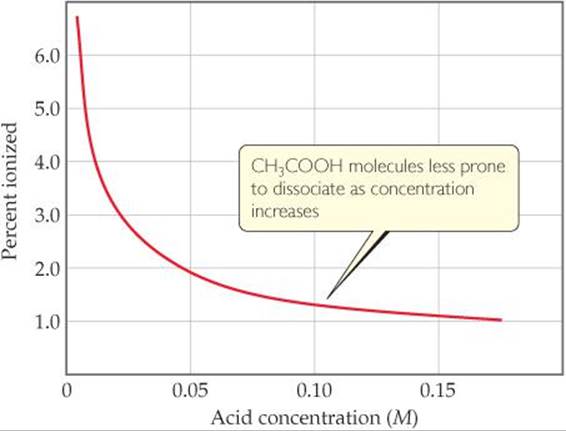
![]() FIGURE 16.11 Effect of concentration on percent ionization in an acetic acid solution.
FIGURE 16.11 Effect of concentration on percent ionization in an acetic acid solution.
SAMPLE EXERCISE 16.13 Using Ka to Calculate Percent Ionization
Calculate the percentage of HF molecules ionized in (a) a 0.10 M HF solution, (b) a 0.010 M HF solution.
SOLUTION
Analyze We are asked to calculate the percent ionization of two HF solutions of different concentration. From Appendix D, we find Ka = 6.8 × 10–4.
Plan We approach this problem as we have previous equilibrium problems. We begin by writing the chemical equation for the equilibrium and tabulating the known and unknown concentrations of all species. We then substitute the equilibrium concentrations into the equilibrium-constant expression and solve for the unknown concentration, that of H+.
Solve
(a) The equilibrium reaction and equilibrium concentrations are as follows:

The equilibrium-constant expression is
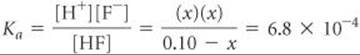
When we try solving this equation using the approximation ![]() (that is, by neglecting the concentration of acid that ionizes), we obtain
(that is, by neglecting the concentration of acid that ionizes), we obtain
![]()
Because this value is greater than 5% of 0.10 M, however, we should work the problem without the approximation. Rearranging our equation and writing it in standard quadratic form, we have
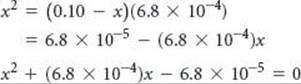
Substituting these values in the standard quadratic formula gives
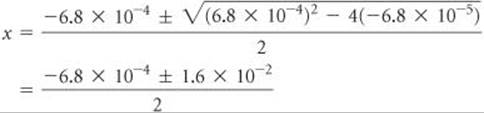
Of the two solutions, only the one that gives a positive value for x is chemically reasonable. Thus,
![]()
From our result, we can calculate the percent of molecules ionized:

(b) Proceeding similarly for the 0.010 M solution, we have
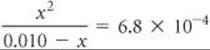
Solving the resultant quadratic expression, we obtain
![]()
The percentage of molecules ionized is
![]()
Comment Notice that if we do not use the quadratic formula, we calculate 8.2% ionization for (a) and 26% ionization for (b). Notice also that in diluting the solution by a factor of 10, the percentage of molecules ionized increases by a factor of 3. This result is in accord with what we see inFigure 16.11. It is also what we expect from Le Châtelier's principle. ![]() (Section 15.7) There are more “particles” or reaction components on the right side of the equation than on the left. Dilution causes the reaction to shift in the direction of the larger number of particles (toward the product side) because this counters the effect of the decreasing concentration of particles.
(Section 15.7) There are more “particles” or reaction components on the right side of the equation than on the left. Dilution causes the reaction to shift in the direction of the larger number of particles (toward the product side) because this counters the effect of the decreasing concentration of particles.
PRACTICE EXERCISE
In Practice Exercise 16.11, we found that the percent ionization of niacin (Ka = 1.5 × 10–5) in a 0.020 M solution is 2.7%. Calculate the percentage of niacin molecules ionized in a solution that is (a) 0.010 M, (b) 1.0 × 10–3M.
Answers: (a) 3.9%, (b) 12%
Polyprotic Acids
Many acids have more than one ionizable H atom. These acids are known as polyprotic acids. For example, with sulfurous acid (H2SO3) we have the successive ionizations
![]()
![]()
Note that the acid-dissociation constants are labeled Ka1 and Ka2. The numbers on the constants refer to the particular proton of the acid that is ionizing. Thus, Ka2 always refers to the equilibrium involving removal of the second proton of a polyprotic acid.
Note that Ka2 for sulfurous acid is much smaller than Ka1. Because of electrostatic attractions, we would expect a positively charged proton to be lost more readily from the neutral H2SO3 molecule than from the negatively charged HSO3– ion. This observation is general: It is always easier to remove the first proton from a polyprotic acid than to remove the second. Similarly, for an acid with three ionizable protons, it is easier to remove the second proton than the third. Thus, the Ka values become successively smaller as successive protons are removed.
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
What is meant by the symbol Ka3 for H3PO4?
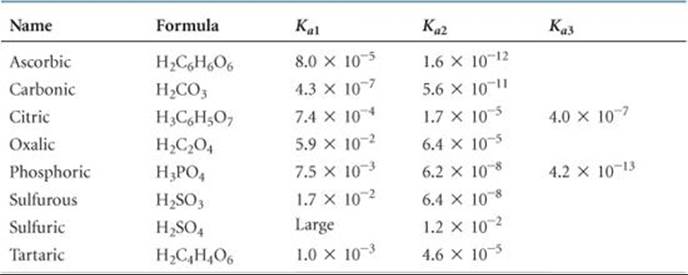
The acid-dissociation constants for common polyprotic acids are listed in ![]() TABLE 16.3, and Appendix D provides a more complete list. The structure of citric acid illustrates the presence of multiple ionizable protons
TABLE 16.3, and Appendix D provides a more complete list. The structure of citric acid illustrates the presence of multiple ionizable protons ![]() FIGURE 16.12.
FIGURE 16.12.
![]() GO FIGURE
GO FIGURE
Citric acid has four hydrogens bonded to oxygen. How does the hydrogen that is not an acidic proton differ from the other three?
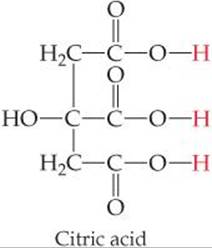
![]() FIGURE 16.12 The structure of the polyprotic acid, citric acid.
FIGURE 16.12 The structure of the polyprotic acid, citric acid.
Notice in Table 16.3 that in most cases the Ka values for successive losses of protons differ by a factor of at least 103. Notice also that the value of Ka1 for sulfuric acid is listed simply as “large.” Sulfuric acid is a strong acid with respect to the removal of the first proton. Thus, the reaction for the first ionization step lies completely to the right:
![]()
However, HSO4– is a weak acid for which Ka2 = 1.2 × 10–2.
For many polyprotic acids Ka1 is much larger than subsequent dissociation constants, in which case the H+(aq) in the solution comes almost entirely from the first ionization reaction. As long as successive Ka values differ by a factor of 103 or more, it is possible to obtain a satisfactory estimate of the pH of polyprotic acid solutions by treating the acids as if they were monoprotic, considering only Ka1.
TABLE 16.3 • Acid-Dissociation Constants of Some Common Polyprotic Acids
SAMPLE EXERCISE 16.14 Calculating the pH of a Polyprotic Acid Solution
The solubility of CO2 in water at 25 °C and 0.1 atm is 0.0037 M. The common practice is to assume that all the dissolved CO2 is in the form of carbonic acid (H2CO3), which is produced in the reaction
![]()
What is the pH of a 0.0037 M solution of H2CO3?
SOLUTION
Analyze We are asked to determine the pH of a 0.0037 M solution of a polyprotic acid.
Plan H2CO3 is a diprotic acid; the two acid-dissociation constants, Ka1 and Ka2 (Table 16.3), differ by more than a factor of 103. Consequently, the pH can be determined by considering only Ka1, thereby treating the acid as if it were a monoprotic acid.
Solve Proceeding as in Sample Exercises 16.12 and 16.13, we can write the equilibrium reaction and equilibrium concentrations as
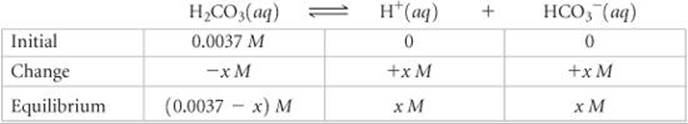
The equilibrium-constant expression is
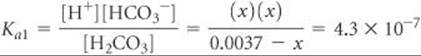
Solving this equation using an equation-solving calculator, we get
![]()
Alternatively, because Ka1 is small, we can make the simplifying approximation that x is small, so that
![]()
Thus,
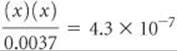
Solving for x, we have
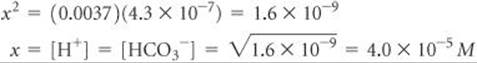
Because we get the same value (to 2 significant figures) our simplifying assumption was justified. The pH is therefore
![]()
Comment If we were asked for [CO32–], we would need to use Ka2. Let's illustrate that calculation. Using our calculated values of [HCO3–] and [H+] and setting [CO32–] = y, we have

Assuming that y is small relative to 4.0 × 10–5, we have
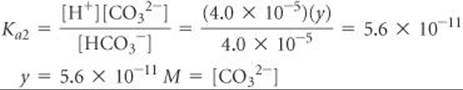
This value is indeed very small compared with 4.0 × 10–5, showing that our assumption was justified. It also shows that the ionization of HCO3– is negligible relative to that of H2CO3, as far as production of H+ is concerned. However, it is the only source of CO32–, which has a very low concentration in the solution. Our calculations thus tell us that in a solution of carbon dioxide in water, most of the CO2 is in the form of CO2 or H2CO3, only a small fraction ionizes to form H+ and HCO3–, and an even smaller fraction ionizes to give CO32–. Notice also that [CO32–] is numerically equal to Ka2.
PRACTICE EXERCISE
(a) Calculate the pH of a 0.020 M solution of oxalic acid (H2C2O4). (See Table 16.3 for Ka1 and Ka2.) (b) Calculate the concentration of oxalate ion, [C2O42–], in this solution.
Answers: (a) pH = 1.80, (b) [C2O42–] = 6.4 × 10–5M