CHEMISTRY THE CENTRAL SCIENCE
16 ACID–BASE EQUILIBRIA
EXERCISES
VISUALIZING CONCEPTS
16.1 (a) Identify the Brønsted–Lowry acid and base in the reaction
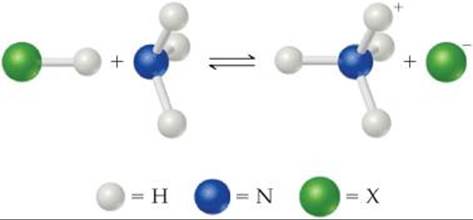
(b) Identify the Lewis acid and base in the reaction. [Sections 16.2 and 16.11]
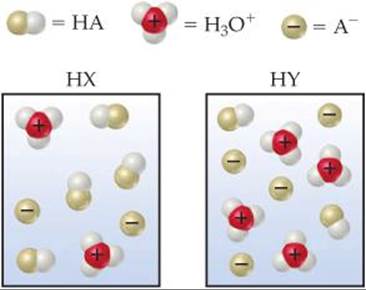
16.2 The following diagrams represent aqueous solutions of two monoprotic acids, HA (A = X or Y). The water molecules have been omitted for clarity. (a) Which is the stronger acid, HX or HY? (b) Which is the stronger base, X– or Y–? (c) If you mix equal concentrations of HX and NaY, will the equilibrium
![]()
lie mostly to the right (Kc > 1) or to the left (Kc < 1)? [Section 16.2]
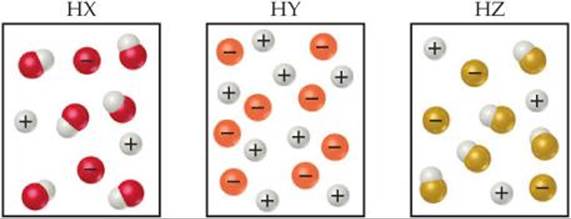
16.3 The following diagrams represent aqueous solutions of three acids, HX, HY, and HZ. The water molecules have been omitted for clarity, and the hydrated proton is represented as H+ rather than H3O+. (a) Which of the acids is a strong acid? Explain. (b) Which acid would have the smallest acid-dissociation constant, Ka? (c) Which solution would have the highest pH? [Sections 16.5 and 16.6]
16.4 In which of the following cases is the approximation that the equilibrium concentration of H+(aq) is small relative to the initial concentration of HA likely to be most valid: (a) initial [HA] = 0.100 M and Ka = 1.0 × 10–6, (b) initial [HA] = 0.100 M and Ka = 1.0 × 10–4, (c) initial [HA] = 1.00 M and Ka = 1.0 × 10–6? [Section 16.6]
16.5 The indicator methyl orange has been added to both of these solutions. Based on the colors, classify each statement as true or false:
(a) The pH of solution A is definitely less than 7.00.
(b) The pH of solution B is definitely greater than 7.00.
(c) The pH of solution B is greater than that of solution A. [Section 16.4]

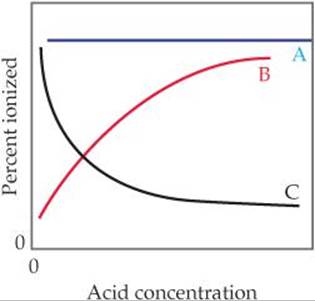
16.6 (a) Which of these three lines represents the effect of concentration on the percent ionization of a weak acid? (b) Explain in qualitative terms why the curve you chose has the shape it does. [Section 16.6]
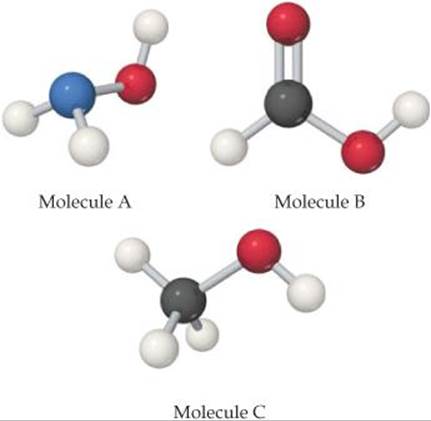
16.7 Each of the three molecules shown here contains an OH group, but one molecule acts as a base, one as an acid, and the third is neither acid nor base. (a) Which one acts as a base? Why does only this molecule act as a base? (b) Which one acts as an acid? (c) Why is the remaining molecule neither acidic nor basic? [Sections 16.6 and 16.7]
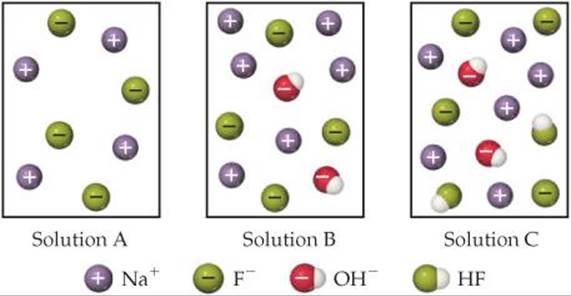
16.8 Which of the following diagrams best represents an aqueous solution of NaF? The water molecules are not shown for clarity. Will this solution be acidic, neutral, or basic? [Section 16.9]
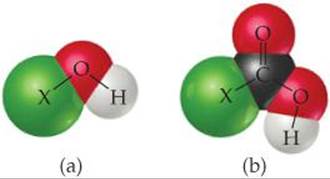
16.9 Consider the molecular models shown here, where X represents a halogen atom. (a) If X is the same atom in both molecules, which one will be more acidic? (b) Does the acidity of each molecule increase or decrease as the electronegativity of the atom X increases? [Section 16.10]
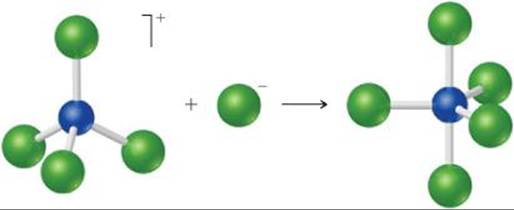
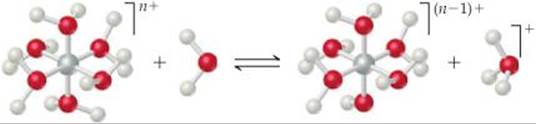
16.10 (a) The following diagram represents the reaction of PCl4+ with Cl–. Draw the Lewis structures for the reactants and products, and identify the Lewis acid and the Lewis base in the reaction.
(b) The following reaction represents a hydrated cation losing a proton. How does the equilibrium constant for the reaction change as the charge of the cation increases? [Sections 16.9 and 16.11]
ARRHENIUS AND BRØNSTED–LOWRY ACIDS AND BASES (sections 16.1 and 16.2)
16.11 Although HCl and H2SO4 have very different properties as pure substances, their aqueous solutions possess many common properties. List some general properties of these solutions, and explain their common behavior in terms of the species present.
16.12 Although pure NaOH and NH3 have very different properties, their aqueous solutions possess many common properties. List some general properties of these solutions, and explain their common behavior in terms of the species present.
______
16.13 (a) What is the difference between the Arrhenius and the Brønsted–Lowry definitions of an acid? (b) NH3(g) and HCl(g) react to form the ionic solid NH4Cl(s). Which substance is the Brønsted–Lowry acid in this reaction? Which is the Brønsted–Lowry base?
16.14 (a) What is the difference between the Arrhenius and the Brønsted–Lowry definitions of a base? (b) Can a substance behave as an Arrhenius base if it does not contain an OH group? Explain.
______
16.15 (a) Give the conjugate base of the following Brønsted–Lowry acids: (i) HIO3, (ii) NH4+. (b) Give the conjugate acid of the following Brønsted–Lowry bases: (i) O2–, (ii) H2PO4–.
16.16 (a) Give the conjugate base of the following Brønsted–Lowry acids: (i) HCOOH, (ii) HPO42–. (b) Give the conjugate acid of the following Brønsted–Lowry bases: (i) SO42–, (ii) CH3NH2.
______
16.17 Designate the Brønsted–Lowry acid and the Brønsted–Lowry base on the left side of each of the following equations, and also designate the conjugate acid and conjugate base of each on the right side:
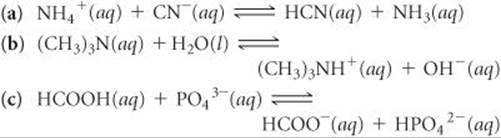
16.18 Designate the Brønsted–Lowry acid and the Brønsted–Lowry base on the left side of each equation, and also designate the conjugate acid and conjugate base of each on the right side.
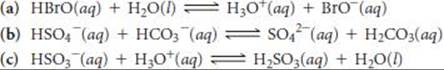
______
16.19 (a) The hydrogen oxalate ion (HC2O4–) is amphiprotic. Write a balanced chemical equation showing how it acts as an acid toward water and another equation showing how it acts as a base toward water. (b) What is the conjugate acid of HC2O4–? What is its conjugate base?
16.20 (a) Write an equation for the reaction in which H2C6H7O5–(aq) acts as a base in H2O(l). (b) Write an equation for the reaction in which H2C6H7O5–(aq) acts as an acid in H2O(l). (c) What is the conjugate acid of H2C6H7O5–(aq)? What is its conjugate base?
______
16.21 Label each of the following as being a strong base, a weak base, or a species with negligible basicity. In each case write the formula of its conjugate acid, and indicate whether the conjugate acid is a strong acid, a weak acid, or a species with negligible acidity: (a) CH3COO–, (b)HCO3–, (c) O2–, (d) Cl–, (e) NH3.
16.22 Label each of the following as being a strong acid, a weak acid, or a species with negligible acidity. In each case write the formula of its conjugate base, and indicate whether the conjugate base is a strong base, a weak base, or a species with negligible basicity: (a) HCOOH, (b)H2, (c) CH4, (d) HF, (e) NH4+.
______
16.23 (a) Which of the following is the stronger Brønsted–Lowry acid, HBrO or HBr? (b) Which is the stronger Brønsted–Lowry base, F– or Cl–? Briefly explain your choices.
16.24 (a) Which of the following is the stronger Brønsted–Lowry acid, HClO3 or HClO2? (b) Which is the stronger Brønsted–Lowry base, HS– or HSO4–? Briefly explain your choices.
______
16.25 Predict the products of the following acid–base reactions, and predict whether the equilibrium lies to the left or to the right of the equation:
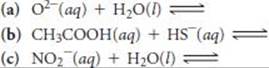
16.26 Predict the products of the following acid–base reactions, and predict whether the equilibrium lies to the left or to the right of the equation:
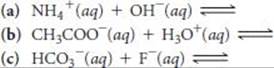
AUTOIONIZATION OF WATER (section 16.3)
16.27 If a neutral solution of water, with pH = 7.00, is heated to 50 °C, the pH drops to 6.63. Does this mean that the concentration of [H+] is greater than the concentration of [OH–]? Explain.
16.28 (a) Write a chemical equation that illustrates the autoionization of water. (b) Write the expression for the ion-product constant for water, Kw. Why is [H2O] absent from this expression? (c) A solution is described as basic. What does this statement mean?
______
16.29 Calculate [H+] for each of the following solutions, and indicate whether the solution is acidic, basic, or neutral: (a) [OH–] = 0.00045 M; (b) [OH–] = 8.8 × 10–9M; (c) a solution in which [OH–] is 100 times greater than [H+].
16.30 Calculate [OH–] for each of the following solutions, and indicate whether the solution is acidic, basic, or neutral: (a) [H+] = 0.0505 M; (b) [H+] = 2.5 × 10–10M; (c) a solution in which [H+] is 1000 times greater than [OH–].
______
16.31 At the freezing point of water (0 °C), Kw = 1.2 × 10–15. Calculate [H+] and [OH–] for a neutral solution at this temperature.
16.32 Deuterium oxide (D2O, where D is deuterium, the hydrogen-2 isotope) has an ion-product constant, Kw, of 8.9 × 10–16 at 20 °C. Calculate [D+] and [OD–] for pure (neutral) D2O at this temperature.
THE pH SCALE (section 16.4)
16.33 By what factor does [H+] change for a pH change of (a) 2.00 units, (b) 0.50 units?
16.34 Consider two solutions, solution A and solution B. [H+] in solution A is 250 times greater than that in solution B. What is the difference in the pH values of the two solutions?
______
16.35 (a) If NaOH is added to water, how does [H+] change? How does pH change? (b) Use the pH values in Figure 16.5 to estimate the pH of a solution with [H+] = 0.0006 M. Is the solution acidic or basic? (c) If the pH of a solution is 5.2, first estimate and then calculate the molar concentrations of H+(aq) and OH–(aq) in the solution.
16.36 (a) If HNO3 is added to water, how does [OH–] change? How does pH change? (b) Use the pH values in Figure 16.5 to estimate the pH of a solution with [OH–] = 0.014 M. Is the solution acidic or basic? (c) If pH = 6.6, first estimate and then calculate the molar concentrations of H+(aq) and OH–(aq) in the solution.
______
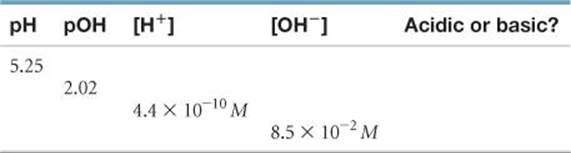
16.37 Complete the following table by calculating the missing entries and indicating whether the solution is acidic or basic.
16.38 Complete the following table by calculating the missing entries. In each case indicate whether the solution is acidic or basic.

______
16.39 The average pH of normal arterial blood is 7.40. At normal body temperature (37 °C), Kw = 2.4 × 10–14. Calculate [H+], [OH–], and pOH for blood at this temperature.
16.40 Carbon dioxide in the atmosphere dissolves in raindrops to produce carbonic acid (H2CO3), causing the pH of clean, unpolluted rain to range from about 5.2 to 5.6. What are the ranges of [H+] and [OH–] in the raindrops?
STRONG ACIDS AND BASES (section 16.5)
16.41 (a) What is a strong acid? (b) A solution is labeled 0.500 M HCl. What is [H+] for the solution? (c) Which of the following are strong acids: HF, HCl, HBr, HI?
16.42 (a) What is a strong base? (b) A solution is labeled 0.035 M Sr(OH)2. What is [OH–] for the solution? (c) Is the following statement true or false? Because Mg(OH)2 is not very soluble, it cannot be a strong base. Explain.
______
16.43 Calculate the pH of each of the following strong acid solutions: (a) 8.5 × 10–3M HBr, (b) 1.52 g of HNO3 in 575 mL of solution, (c) 5.00 mL of 0.250 M HClO4 diluted to 50.0 mL, (d) a solution formed by mixing 10.0 mL of 0.100 M HBr with 20.0 mL of 0.200 M HCl.
16.44 Calculate the pH of each of the following strong acid solutions: (a) 0.0167 M HNO3, (b) 0.225 g of HClO3 in 2.00 L of solution, (c) 15.00 mL of 1.00 M HCl diluted to 0.500 L, (d) a mixture formed by adding 50.0 mL of 0.020 M HCl to 125 mL of 0.010 M HI.
______
16.45 Calculate [OH–] and pH for (a) 1.5 × 10–3M Sr(OH2), (b) 2.250 g of LiOH in 250.0 mL of solution, (c) 1.00 mL of 0.175 M NaOH diluted to 2.00 L, (d) a solution formed by adding 5.00 mL of 0.105 M KOH to 15.0 mL of 9.5 × 10–2M Ca(OH)2.
16.46 Calculate [OH–] and pH for each of the following strong base solutions: (a) 0.182 M KOH, (b) 3.165 g of KOH in 500.0 mL of solution, (c) 10.0 mL of 0.0105 M Ca(OH)2 diluted to 500.0 mL, (d) a solution formed by mixing 20.0 mL of 0.015 M Ba(OH)2 with 40.0 mL of 8.2 × 10–3M NaOH.
______
16.47 Calculate the concentration of an aqueous solution of NaOH that has a pH of 11.50.
16.48 Calculate the concentration of an aqueous solution of Ca(OH)2 that has a pH of 10.05.
WEAK ACIDS (section 16.6)
16.49 Write the chemical equation and the Ka expression for the ion-ization of each of the following acids in aqueous solution. First show the reaction with H+(aq) as a product and then with the hydronium ion: (a) HBrO2, (b) C2H5COOH.
16.50 Write the chemical equation and the Ka expression for the acid dissociation of each of the following acids in aqueous solution. First show the reaction with H+(aq) as a product and then with the hydronium ion: (a) C6H5COOH, (b) HCO3–.
______
16.51 Lactic acid (CH3CH(OH)COOH) has one acidic hydrogen. A 0.10 M solution of lactic acid has a pH of 2.44. Calculate Ka.
16.52 Phenylacetic acid (C6H5CH2COOH) is one of the substances that accumulates in the blood of people with phenylketonuria, an inherited disorder that can cause mental retardation or even death. A 0.085 M solution of C6H5CH2COOH has a pH of 2.68. Calculate the Ka value for this acid.
______
16.53 A 0.100 M solution of chloroacetic acid (ClCH2COOH) is 11.0% ionized. Using this information, calculate [ClCH2COO–], [H+], [ClCH2COOH], and Ka for chloroacetic acid.
16.54 A 0.100 M solution of bromoacetic acid (BrCH2COOH) is 13.2% ionized. Calculate [H+], [BrCH2COO–], [BrCH2COOH] and Ka for bromoacetic acid.
______
16.55 A particular sample of vinegar has a pH of 2.90. If acetic acid is the only acid that vinegar contains (Ka = 1.8 × 10–5), calculate the concentration of acetic acid in the vinegar.
16.56 If a solution of HF (Ka = 6.8 × 10–4) has a pH of 3.65, calculate the concentration of hydrofluoric acid.
______
16.57 The acid-dissociation constant for benzoic acid (C6H5COOH) is 6.3 × 10–5. Calculate the equilibrium concentrations of H3O+, C6H5COO–, and C6H5COOH in the solution if the initial concentration of C6H5COOH is 0.050 M.
16.58 The acid-dissociation constant for chlorous acid (HClO2) is 1.1 × 10–2. Calculate the concentrations of H3O+, ClO2–, and HClO2 at equilibrium if the initial concentration of HClO2 is 0.0125 M.
______
16.59 Calculate the pH of each of the following solutions (Ka and Kb values are given in Appendix D): (a) 0.095 M propionic acid (C2H5COOH), (b) 0.100 M hydrogen chromate ion (HCrO4–), (c) 0.120 M pyridine (C5H5N).
16.60 Determine the pH of each of the following solutions (Ka and Kb values are given in Appendix D): (a) 0.095 M hypochlorous acid, (b) 0.0085 M hydrazine, (c) 0.165 M hydroxylamine.
______
16.61 Saccharin, a sugar substitute, is a weak acid with pKa = 2.32 at 25 °C. It ionizes in aqueous solution as follows:
![]()
What is the pH of a 0.10 M solution of this substance?
16.62 The active ingredient in aspirin is acetylsalicylic acid (HC9H7O4), a monoprotic acid with Ka = 3.3 × 10–4 at 25 °C. What is the pH of a solution obtained by dissolving two extra-strength aspirin tablets, containing 500 mg of acetylsalicylic acid each, in 250 mL of water?
______
16.63 Calculate the percent ionization of hydrazoic acid (HN3) in solutions of each of the following concentrations (Ka is given in Appendix D): (a) 0.400 M, (b) 0.100 M, (c) 0.0400 M.
16.64 Calculate the percent ionization of propionic acid (C2H5COOH) in solutions of each of the following concentrations (Ka is given in Appendix D): (a) 0.250 M, (b) 0.0800 M, (c) 0.0200 M.
______
16.65 Show that for a weak acid, the percent ionization should vary as the inverse square root of the acid concentration.
16.66 For solutions of a weak acid, a graph of pH versus the logarithm of the initial acid concentration should be a straight line. What is the magnitude of the slope of that line?
______
16.67 Citric acid, which is present in citrus fruits, is a triprotic acid (Table 16.3). Calculate the pH of a 0.040 M solution of citric acid. Explain any approximations or assumptions you make in your calculations. Is the concentration of citrate ion (C6H5O7)3– equal to, less than, or greater than the H+ ion concentration?
16.68 Tartaric acid is found in many fruits, including grapes, and is partially responsible for the dry texture of certain wines. Calculate the pH and the tartarate ion (C4H4O62–) concentration for a 0.250 M solution of tartaric acid, for which the acid-dissociation constants are listed inTable 16.3. Explain any approximations or assumptions that you make in your calculation.
WEAK BASES (section 16.7)
16.69 Consider the base hydroxylamine, NH2OH. (a) What is the conjugate acid of hydroxylamine? (b) When it acts as a base, which atom in hydroxylamine accepts a proton? (c) There are two atoms in hydroxylamine that have nonbonding electron pairs that could act as proton acceptors. Use Lewis structures and formal charges ![]() (Section 8.5) to rationalize why one of these two atoms is a much better proton acceptor than the other.
(Section 8.5) to rationalize why one of these two atoms is a much better proton acceptor than the other.
16.70 The hypochlorite ion, ClO–, acts as a weak base. (a) Is ClO– a stronger or weaker base than hydroxylamine? (b) When ClO– acts as a base, which atom, Cl or O, acts as the proton acceptor? (c) Can you use formal charges to rationalize your answer to part (b)?
______
16.71 Write the chemical equation and the Kb expression for the reaction of each of the following bases with water: (a) dimethylamine, (CH3)2NH; (b) carbonate ion, CO32–; (c) formate ion, CHO2–.
16.72 Write the chemical equation and the Kb expression for the reaction of each of the following bases with water: (a) propylamine, C3H7NH2; (b) monohydrogen phosphate ion, HPO42–; (c) benzoate ion, C6H5CO2–.
______
16.73 Calculate the molar concentration of OH– ions in a 0.075 M solution of ethylamine (C2H5NH2; Kb = 6.4 × 10–4). Calculate the pH of this solution.
16.74 Calculate the molar concentration of OH– ions in a 0.724 M solution of hypobromite ion (BrO–; Kb = 4.0 × 10–6). What is the pH of this solution?
______
16.75 Ephedrine, a central nervous system stimulant, is used in nasal sprays as a decongestant. This compound is a weak organic base:
![]()
A 0.035 M solution of ephedrine has a pH of 11.33. (a) What are the equilibrium concentrations of C10H15ON, C10H15ONH+, and OH–? (b) Calculate Kb, for ephedrine.
16.76 Codeine (C18H21NO3) is a weak organic base. A 5.0 × 10–3M solution of codeine has a pH of 9.95. Calculate the value of Kb for this substance. What is the pKb for this base?
THE Ka–Kb RELATIONSHIP; ACID–BASE PROPERTIES OF SALTS (sections 16.8 and 16.9)
16.77 Although the acid-dissociation constant for phenol (C6H5OH) is listed in Appendix D, the base-dissociation constant for the phenolate ion (C6H5O–) is not. (a) Explain why it is not necessary to list both Ka for phenol and Kb for the phenolate ion. (b) Calculate Kb for the phenolate ion. (c) Is the phenolate ion a weaker or stronger base than ammonia?
16.78 Use the acid-dissociation constants in Table 16.3 to arrange these oxyanions from strongest base to weakest: SO42–, CO32–, SO32–, and PO43–.
______
16.79 (a) Given that Ka for acetic acid is 1.8 × 10–5 and that for hypochlorous acid is 3.0 × 10–8, which is the stronger acid? (b) Which is the stronger base, the acetate ion or the hypochlorite ion? (c) Calculate Kb values for CH3COO– and ClO–.
16.80 (a) Given that Kb for ammonia is 1.8 × 10–5 and that for hydroxylamine is 1.1 × 10–8, which is the stronger base? (b) Which is the stronger acid, the ammonium ion or the hydroxylammonium ion? (c) Calculate Ka values for NH4+ and H3NOH+.
______
16.81 Using data from Appendix D, calculate [OH–] and pH for each of the following solutions: (a) 0.10 M NaBrO, (b) 0.080 M NaHS, (c) a mixture that is 0.10 M in NaNO2 and 0.20 M in Ca(NO2)2.
16.82 Using data from Appendix D, calculate [OH–] and pH for each of the following solutions: (a) 0.105 M NaF, (b) 0.035 M Na2S, (c) a mixture that is 0.045 M in CH3COONa and 0.055 M in (CH3COO)2Ba.
______
16.83 Predict whether aqueous solutions of the following compounds are acidic, basic, or neutral: (a) NH4Br, (b) FeCl3, (c) Na2CO3, (d) KClO4, (e) NaHC2O4.
16.84 Predict whether aqueous solutions of the following substances are acidic, basic, or neutral: (a) AlCl3, (b) NaBr, (c) NaClO, (d) [CH3NH3]NO3, (e) Na2SO3.
______
16.85 An unknown salt is either NaF, NaCl, or NaOCl. When 0.050 mol of the salt is dissolved in water to form 0.500 L of solution, the pH of the solution is 8.08. What is the identity of the salt?
16.86 An unknown salt is either KBr, NH4Cl, KCN, or K2CO3. If a 0.100 M solution of the salt is neutral, what is the identity of the salt?
ACID–BASE CHARACTER AND CHEMICAL STRUCTURE (section 16.10)
16.87 How does the acid strength of an oxyacid depend on (a) the electronegativity of the central atom; (b) the number of non-protonated oxygen atoms in the molecule?
16.88 (a) Why is NH3 a stronger base than H2O? (b) Why is NH3 a stronger base than CH4?
______
16.89 Explain the following observations: (a) HNO3 is a stronger acid than HNO2; (b) H2S is a stronger acid than H2O; (c) H2SO4 is a stronger acid than HSO4–; (d) H2SO4 is a stronger acid than H2SeO4; (e) CCl3COOH is a stronger acid than CH3COOH.
16.90 Explain the following observations: (a) HCl is a stronger acid than H2S; (b) H3PO4 is a stronger acid than H3AsO4; (c) HBrO3 is a stronger acid than HBrO2; (d) H2C2O4 is a stronger acid than HC2O4–; (e) benzoic acid (C6H5COOH) is a stronger acid than phenol (C6H5OH).
______
16.91 Based on their compositions and structures and on conjugate acid–base relationships, select the stronger base in each of the following pairs: (a) BrO– or ClO–, (b) BrO– or BrO2–, (c) HPO42– or H2PO4–.
16.92 Based on their compositions and structures and on conjugate acid–base relationships, select the stronger base in each of the following pairs: (a) NO3– or NO2–, (b) PO43– or AsO43–, (c) HCO3– or CO32–.
______
16.93 Indicate whether each of the following statements is true or false. For each statement that is false, correct the statement to make it true. (a) In general, the acidity of binary acids increases from left to right in a given row of the periodic table. (b) In a series of acids that have the same central atom, acid strength increases with the number of hydrogen atoms bonded to the central atom. (c) Hydrotelluric acid (H2Te) is a stronger acid than H2S because Te is more electronegative than S.
16.94 Indicate whether each of the following statements is true or false. For each statement that is false, correct the statement to make it true. (a) Acid strength in a series of H—X molecules increases with increasing size of X. (b) For acids of the same general structure but differing electronegativities of the central atoms, acid strength decreases with increasing electronegativity of the central atom. (c) The strongest acid known is HF because fluorine is the most electronegative element.
LEWIS ACIDS AND BASES (section 16.11)
16.95 If a substance is an Arrhenius base, is it necessarily a Brønsted–Lowry base? Is it necessarily a Lewis base? Explain.
16.96 If a substance is a Lewis acid, is it necessarily a Brønsted–Lowry acid? Is it necessarily an Arrhenius acid? Explain.
______
16.97 Identify the Lewis acid and Lewis base among the reactants in each of the following reactions:
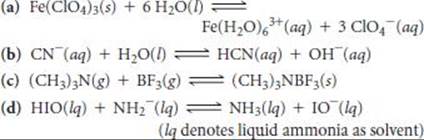
16.98 Identify the Lewis acid and Lewis base in each of the following reactions:
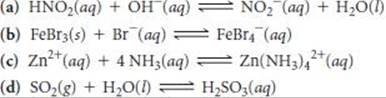
______
16.99 Predict which member of each pair produces the more acidic aqueous solution: (a) K+ or Cu2+, (b) Fe2+ or Fe3+, (c) Al3+ or Ga3+. Explain.
16.100 Which member of each pair produces the more acidic aqueous solution: (a) ZnBr2 or CdCl2, (b) CuCl or Cu(NO3)2, (c) Ca(NO3)2 or NiBr2? Explain.
ADDITIONAL EXERCISES
16.101 Triethylamine, (C2H5)3N, has a pKb value of 2.99. Is triethylamine a stronger base than ammonia, NH3?
16.102 Indicate whether each of the following statements is correct or incorrect. For those that are incorrect, explain why they are wrong.
(a) Every Brønsted–Lowry acid is also a Lewis acid.
(b) Every Lewis acid is also a Brønsted–Lowry acid.
(c) Conjugate acids of weak bases produce more acidic solutions than conjugate acids of strong bases.
(d) K+ ion is acidic in water because it causes hydrating water molecules to become more acidic.
(e) The percent ionization of a weak acid in water increases as the concentration of acid decreases.
16.103 Use Figure 16.3 to predict whether the equilibrium lies to the right or to the left in the following reactions:
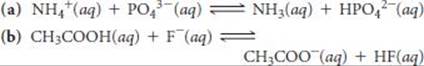
16.104 The odor of fish is due primarily to amines, especially methy-lamine (CH3NH2). Fish is often served with a wedge of lemon, which contains citric acid. The amine and the acid react forming a product with no odor, thereby making the less-than-fresh fish more appetizing. Using data from Appendix D, calculate the equilibrium constant for the reaction of citric acid with methylamine, if only the first proton of the citric acid (Ka1) is important in the neutralization reaction.
16.105 Hemoglobin plays a part in a series of equilibria involving protonation-deprotonation and oxygenation-deoxygenation. The overall reaction is approximately as follows:
![]()
where Hb stands for hemoglobin and HbO2 for oxyhemoglobin. (a) The concentration of O2 is higher in the lungs and lower in the tissues. What effect does high [O2] have on the position of this equilibrium? (b) The normal pH of blood is 7.4. Is the blood acidic, basic, or neutral? (c) If the blood pH is lowered by the presence of large amounts of acidic metabolism products, a condition known as acidosis results. What effect does lowering blood pH have on the ability of hemoglobin to transport O2?
[16.106] Calculate the pH of a solution made by adding 2.50 g of lithium oxide (Li2O) to enough water to make 1.500 L of solution.
16.107 Which of the following solutions has the higher pH? (a) a 0.1 M solution of a strong acid or a 0.1 M solution of a weak acid, (b) a 0.1 M solution of an acid with Ka = 2 × 10–3 or one with Ka = 8 × 10–6, (c) a 0.1 M solution of a base with pKb = 4.5 or one with pKb = 6.5.
16.108 What is the pH of a solution that is 2.5 × 10–9 in NaOH? Does your answer make sense? What assumption do we normally make that is not valid in this case?
16.109 Caproic acid (C5H11COOH) is found in small amounts in coconut and palm oils and is used in making artificial flavors. A saturated solution of the acid contains 11 g/L and has a pH of 2.94. Calculate Ka for the acid.
16.110 Butyric acid is responsible for the foul smell of rancid butter. The pKa of butyric acid is 4.84. (a) Calculate the pKb for the butyrate ion. (b) Calculate the pH of a 0.050 M solution of butyric acid. (c) Calculate the pH of a 0.050 M solution of sodium butyrate.
16.111 Arrange the following 0.10 M solutions in order of increasing acidity (decreasing pH): (i) NH4NO3, (ii) NaNO3, (iii) CH3COONH4, (iv) NaF, (v) CH3COONa.
[16.112] Many moderately large organic molecules containing basic nitrogen atoms are not very soluble in water as neutral molecules, but they are frequently much more soluble as their acid salts. Assuming that pH in the stomach is 2.5, indicate whether each of the following compounds would be present in the stomach as the neutral base or in the protonated form: nicotine, Kb = 7 × 10–7; caffeine, Kb = 4 × 10–14; strychnine, Kb = 1 × 10–6; quinine, Kb = 1.1 × 10–6.
[16.113] The amino acid glycine (H2N—CH2—COOH) can participate in the following equilibria in water:
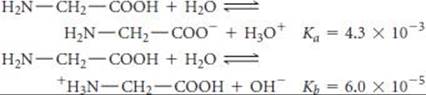
(a) Use the values of Ka and Kb to estimate the equilibrium constant for the intramolecular proton transfer to form a zwitterion:
![]()
What assumptions did you need to make? (b) What is the pH of a 0.050 M aqueous solution of glycine? (c) What would be the predominant form of glycine in a solution with pH 13? With pH 1?
16.114 The structural formula for acetic acid is shown in Table 16.2. Replacing hydrogen atoms on the carbon with chlorine atoms causes an increase in acidity, as follows:

Using Lewis structures as the basis of your discussion, explain the observed trend in acidities in the series. Calculate the pH of a 0.010 M solution of each acid.
INTEGRATIVE EXERCISES
16.115 Calculate the number of H+ (aq) ions in 1.0 mL of pure water at 25 °C.
16.116 How many milliliters of concentrated hydrochloric acid solution (36.0% HCl by mass, density = 1.18g/mL) are required to produce 10.0 L of a solution that has a pH of 2.05?
16.117 The volume of an adult's stomach ranges from about 50 mL when empty to 1 L when full. If the stomach volume is 400 mL and its contents have a pH of 2, how many moles of H+ does the stomach contain? Assuming that all the H+ comes from HCl, how many grams of sodium hydrogen carbonate will totally neutralize the stomach acid?
16.118 Atmospheric CO2 levels have risen by nearly 20% over the past 40 years from 315 ppm to 380 ppm. (a) Given that the average pH of clean, unpolluted rain today is 5.4, determine the pH of unpolluted rain 40 years ago. Assume that carbonic acid (H2CO3) formed by the reaction of CO2 and water is the only factor influencing pH.
![]()
(b) What volume of CO2 at 25°C and 1.0 atm is dissolved in a 20.0-L bucket of today's rainwater?
16.119 In many reactions the addition of AlCl3 produces the same effect as the addition of H+. (a) Draw a Lewis structure for AlCl3 in which no atoms carry formal charges, and determine its structure using the VSEPR method. (b) What characteristic is notable about the structure in part (a) that helps us understand the acidic character of AlCl3? (c) Predict the result of the reaction between AlCl3 and NH3 in a solvent that does not participate as a reactant. (d) Which acid–base theory is most suitable for discussing the similarities between AlCl3 and H+?
16.120 What is the boiling point of a 0.10 M solution of NaHSO4 if the solution has a density of 1.002 g/mL?
16.121 Use average bond enthalpies from Table 8.4 to estimate the enthalpies of the following gas-phase reactions:
![]()
Are both reactions exothermic? How do these values relate to the different strengths of hydrofluoric and hydrochloric acid?
[16.122] Cocaine is a weak organic base whose molecular formula is C17H21NO4. An aqueous solution of cocaine was found to have a pH of 8.53 and an osmotic pressure of 52.7 torr at 15 °C. Calculate Kb for cocaine.
[16.123] The iodate ion is reduced by sulfite according to the following reaction:
![]()
The rate of this reaction is found to be first order in IO3–, first order in SO32–, and first order in H+. (a) Write the rate law for the reaction. (b) By what factor will the rate of the reaction change if the pH is lowered from 5.00 to 3.50? Does the reaction proceed more quickly or more slowly at the lower pH? (c) By using the concepts discussed in Section 14.6, explain how the reaction can be pH-dependent even though H+ does not appear in the overall reaction.
16.124 (a) Using dissociation constants from Appendix D, determine the value for the equilibrium constant for each of the following reactions.
![]()
(b) We usually use single arrows for reactions when the forward reaction is appreciable (K much greater than 1) or when products escape from the system, so that equilibrium is never established. If we follow this convention, which of these equilibria might be written with a single arrow?