CHEMISTRY THE CENTRAL SCIENCE
17 ADDITIONAL ASPECTS OF AQUEOUS EQUILIBRIA
17.6 PRECIPITATION AND SEPARATION OF IONS
Equilibrium can be achieved starting with the substances on either side of a chemical equation. For example, the equilibrium that exists between BaSO4(s), Ba2+(aq), and SO42–(aq) (Equation 17.15), can be achieved either by starting with BaSO4(s) or by starting with solutions containing Ba2+ and SO42–. If we mix, say, a BaCl2 aqueous solution and a Na2SO4 aqueous solution, BaSO4 precipitates if the product of the initial ion concentrations, Q = [Ba2+][SO42–], is greater than Ksp.
The use of the reaction quotient Q to determine the direction in which a reaction must proceed to reach equilibrium was discussed in Section 15.6. The possible relationships between Q and Ksp are as follows:
• If Q > Ksp, precipitation occurs, reducing ion concentrations until Q = Ksp.
• If Q = Ksp, equilibrium exists (saturated solution).
• If Q < Ksp, solid dissolves, increasing ion concentrations until Q = Ksp.
SAMPLE EXERCISE 17.15 Predicting Whether a Precipitate Forms
Does a precipitate form when 0.10 L of 8.0 × 10–3M Pb(NO3)2 is added to 0.40 L of 5.0 × 10–3M Na2SO4?
SOLUTION
Analyze The problem asks us to determine whether a precipitate forms when two salt solutions are combined.
Plan We should determine the concentrations of all ions just after the solutions are mixed and compare the value of Q with Ksp for any potentially insoluble product. The possible metathesis products are PbSO4 and NaNO3. Like all sodium salts NaNO3 is soluble, but PbSO4 has a Ksp of 6.3 × 10–7 (Appendix D) and will precipitate if the Pb+2 and SO42– concentrations are high enough for Q to exceed Ksp.
Solve When the two solutions are mixed, the volume is 0.10 L + 0.40 L = 0.50 L. The number of moles of Pb2+ in 0.10 L of 8.0 × 10–3M Pb(NO3)2 is
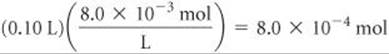
The concentration of Pb2+ in the 0.50-L mixture is therefore
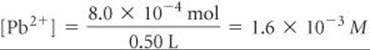
The number of moles of SO42– in 0.40 L of 5.0 × 10–3M Na2SO4 is
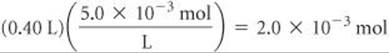
Therefore
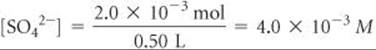
and
![]()
Because Q > Ksp, PbSO4 precipitates.
PRACTICE EXERCISE
Does a precipitate form when 0.050 L of 2.0 × 10–2M NaF is mixed with 0.010 L of 1.0 × 10–2M Ca(NO3)2?
Answer: Yes, CaF2 precipitates because Q = 4.6 × 10–8 is larger than Ksp = 3.9 × 10–11
Selective Precipitation of Ions
Ions can be separated from each other based on the solubilities of their salts. Consider a solution containing both Ag+ and Cu2+. If HCl is added to the solution, AgCl (Ksp = 1.8 × 10–10) precipitates, while Cu2+ remains in solution because CuCl2 is soluble. Separation of ions in an aqueous solution by using a reagent that forms a precipitate with one or more (but not all) of the ions is called selective precipitation.
SAMPLE EXERCISE 17.16 Calculating Ion Concentrations for Precipitation
A solution contains 1.0 × 10–2M Ag+ and 2.0 × 10–2M Pb2+. When Cl– is added, both AgCl (Ksp = 1.8 × 10–10) and PbCl2 (Ksp = 1.7 × 10–5) can precipitate. What concentration of Cl– is necessary to begin the precipitation of each salt? Which salt precipitates first?
SOLUTION
Analyze We are asked to determine the concentration of Cl– necessary to begin the precipitation from a solution containing Ag+ and Pb2+ ions, and to predict which metal chloride will begin to precipitate first.
Plan We are given Ksp values for the two precipitates. Using these and the metal ion concentrations, we can calculate what Cl– concentration is necessary to precipitate each salt. The salt requiring the lower Cl– ion concentration precipitates first.
Solve For AgCl we have Ksp = [Ag+][Cl–] = 1.8 × 10–10
Because [Ag+] = 1.0 × 10–2M, the greatest concentration of Cl– that can be present without causing precipitation of AgCl can be calculated from the Ksp expression:
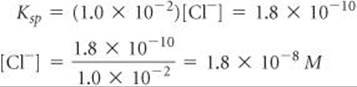
Any Cl– in excess of this very small concentration will cause AgCl to precipitate from solution. Proceeding similarly for PbCl2, we have
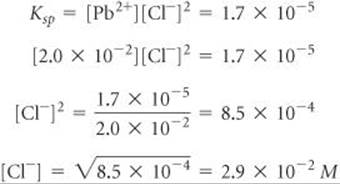
Thus, a concentration of Cl– in excess of 2.9 × 10–2M causes PbCl2 to precipitate.
Comparing the Cl– concentration required to precipitate each salt, we see that as Cl– is added, AgCl precipitates first because it requires a much smaller concentration of Cl–. Thus, Ag+can be separated from Pb2+ by slowly adding Cl– so that the chloride ion concentration remains between 1.8 × 10–8M and 2.9 × 10–2M.
Comment Precipitation of AgCl will keep the Cl– concentration low until the number of moles of Cl– added exceeds the number of moles of Ag+ in the solution. Once past this point, [Cl–] rises sharply and PbCl2 will soon begin to precipitate.
PRACTICE EXERCISE
A solution consists of 0.050 M Mg2+ and Cu2+. Which ion precipitates first as OH– is added? What concentration of OH– is necessary to begin the precipitation of each cation? [Ksp = 1.8 × 10–11 for Mg(OH)2, and Ksp = 4.8 × 10–20 for Cu(OH)2.]
Answer: Cu(OH)2 precipitates first, beginning when [OH–] > 1.5 × 10–9M. Mg(OH)2 begins to precipitate when [OH–] > 1.9 × 10–5M.
![]() GO FIGURE
GO FIGURE
What would happen if the pH were raised to 8 first and then H2S were added?
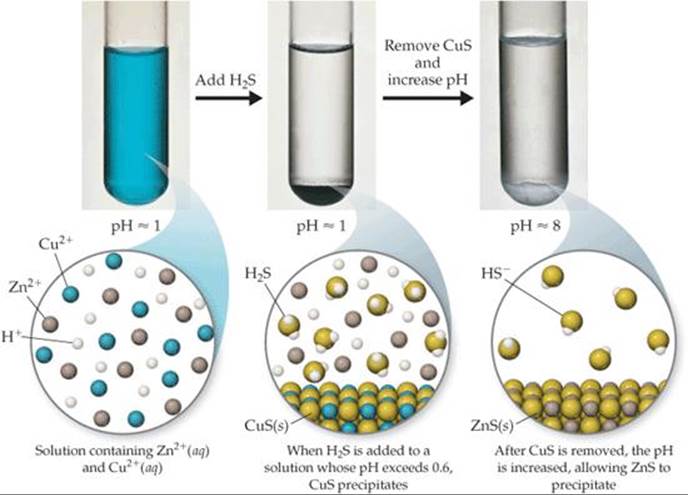
![]() FIGURE 17.22 Selective precipitation. In this example Cu2+ ions are separated from Zn2+ ions.
FIGURE 17.22 Selective precipitation. In this example Cu2+ ions are separated from Zn2+ ions.
Sulfide ion is often used to separate metal ions because the solubilities of sulfide salts span a wide range and depend greatly on solution pH. For example, Cu2+ and Zn2+ can be separated by bubbling H2S gas through an acidified solution containing these two cations. Because CuS (Ksp= 6 × 10–37) is less soluble than ZnS (Ksp = 2 × 10–25), CuS precipitates from an acidified solution pH ≈ 1 while ZnS does not (![]() FIGURE 17.22):
FIGURE 17.22):
![]()
The CuS can be separated from the Zn2+ solution by filtration. The CuS can then be dissolved by raising the concentration of H+ even further, shifting the equilibrium of Equation 17.27 to the left.