CHEMISTRY THE CENTRAL SCIENCE
18 CHEMISTRY OF THE ENVIRONMENT
EXERCISES
VISUALIZING CONCEPTS
18.1 At 273 K and 1 atm pressure, one mole of an ideal gas occupies 22.4 L. ![]() (Section 10.4) (a) Looking back at Figure 18.1, do you predict that 1 mole of an ideal gas in the middle of the stratosphere would occupy a greater or smaller volume than 22.4 L? (b) Looking at Figure 18.1, we see that the temperature is lower at 85 km altitude than at 50 km. Does this mean that one mole of an ideal gas would occupy less volume at 85 km than at 50 km? Explain. (c) In which parts of the atmosphere would you expect gases to behave most ideally (ignoring any photochemical reactions)? [Section 18.1]
(Section 10.4) (a) Looking back at Figure 18.1, do you predict that 1 mole of an ideal gas in the middle of the stratosphere would occupy a greater or smaller volume than 22.4 L? (b) Looking at Figure 18.1, we see that the temperature is lower at 85 km altitude than at 50 km. Does this mean that one mole of an ideal gas would occupy less volume at 85 km than at 50 km? Explain. (c) In which parts of the atmosphere would you expect gases to behave most ideally (ignoring any photochemical reactions)? [Section 18.1]
18.2 Molecules in the upper atmosphere tend to contain double and triple bonds rather than single bonds. Suggest an explanation. [Section 18.1]
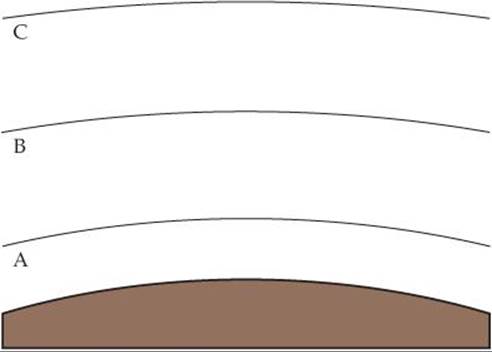
18.3 The figure shows the three lowest regions of Earth's atmosphere. (a) Name each and indicate the approximate elevations at which the boundaries occur. (b) In which region is ozone a pollutant? In which region does it filter UV solar radiation? (c) In which region is infrared radiation from Earth's surface most strongly reflected back? (d) An aurora borealis is due to excitation of atoms and molecules in the atmosphere 55–95 km above Earth's surface. Which regions in the figure are involved in an aurora borealis? (e) Compare the changes in relative concentrations of water vapor and carbon dioxide with increasing elevation in these three regions.
18.4 You are working with an artist who has been commissioned to make a sculpture for a big city in the eastern United States. The artist is wondering what material to use to make her sculpture because she has heard that acid rain in the eastern United States might destroy it over time. You take samples of granite, marble, bronze, and other materials, and place them outdoors for a long time in the big city. You periodically examine the appearance and measure the mass of the samples. (a) What observations would lead you to conclude that one or more of the materials are well-suited for the sculpture? (b) What chemical process (or processes) is (are) the most likely responsible for any observed changes in the materials? [Section 18.2]
18.5 Where does the energy come from to evaporate the estimated 425,000 km3 of water that annually leaves the oceans? [Section 18.3]
18.6 Distinguish among salt water, freshwater, and groundwater. [Section 18.3]
18.7 How does carbon dioxide interact with the world ocean? [Section 18.3]
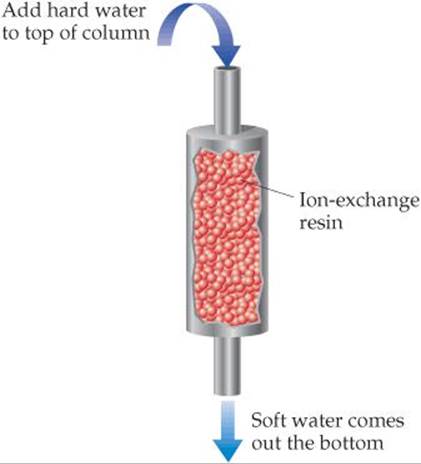
18.8 The following picture represents an ion-exchange column, in which water containing “hard” ions, such as Ca2+, is added to the top of the column, and water containing “soft” ions, such as Na+, comes out the bottom. Explain what is happening in the column. [Section 18.4]
18.9 Describe the basic goals of green chemistry. [Section 18.5]
18.10 One mystery in environmental science is the imbalance in the “carbon dioxide budget.” Considering only human activities, scientists have estimated that 1.6 billion metric tons of CO2 is added to the atmosphere every year because of deforestation (plants use CO2, and fewer plants will leave more CO2 in the atmosphere). Another 5.5 billion tons per year is put into the atmosphere because of burning fossil fuels. It is further estimated (again, considering only human activities) that the atmosphere actually takes up about 3.3 billion tons of this CO2 per year, while the oceans take up 2 billion tons per year, leaving about 1.8 billion tons of CO2 per year unaccounted for. This “missing” CO2 is assumed to be taken up by the “land.” What do you think might be happening? [Sections 18.1–18.3]
EARTH'S ATMOSPHERE (section 18.1)
18.11 (a) What is the primary basis for the division of the atmosphere into different regions? (b) Name the regions of the atmosphere, indicating the altitude interval for each one.
18.12 (a) How are the boundaries between the regions of the atmosphere determined? (b) Explain why the stratosphere, which is more than 20 miles thick, has a smaller total mass than the troposphere, which is less than 10 miles thick.
______
18.13 Air pollution in the Mexico City metropolitan area is among the worst in the world. The concentration of ozone in Mexico City has been measured at 441 ppb (0.441 ppm). Mexico City sits at an altitude of 7400 feet, which means its atmospheric pressure is only 0.67 atm. (a)Calculate the partial pressure of ozone at 441 ppb if the atmospheric pressure is 0.67 atm. (b) How many ozone molecules are in 1.0 L of air in Mexico City? Assume T = 25 °C.
18.14. From the data in Table 18.1, calculate the partial pressures of carbon dioxide and argon when the total atmospheric pressure is 1.05 bar.
______
18.15 The average concentration of carbon monoxide in air in an Ohio city in 2006 was 3.5 ppm. Calculate the number of CO molecules in 1.0 L of this air at a pressure of 759 torr and a temperature of 22 °C.
18.16 (a) From the data in Table 18.1, what is the concentration of neon in the atmosphere in ppm? (b) What is the concentration of neon in the atmosphere in molecules per L, assuming an atmospheric pressure of 730 torr and a temperature of 296 K?
______
18.17 The dissociation energy of a carbon–bromine bond is typically about 210 kJ/mol. (a) What is the maximum wavelength of photons that can cause C—Br bond dissociation? (b) Which kind of electromagnetic radiation—ultraviolet, visible, or infrared—does the wavelength you calculated in part (a) correspond to?
18.18 In CF3Cl the C—Cl bond-dissociation energy is 339 kJ/mol. In CCl4 the C—Cl bond-dissociation energy is 293 kJ/mol. What is the range of wavelengths of photons that can cause C—Cl bond rupture in one molecule but not in the other?
______
18.19 (a) Distinguish between photodissociation and photoionization. (b) Use the energy requirements of these two processes to explain why photodissociation of oxygen is more important than photoionization of oxygen at altitudes below about 90 km.
18.20 Why is the photodissociation of N2 in the atmosphere relatively unimportant compared with the photodissociation of O2?
HUMAN ACTIVITIES AND EARTH'S ATMOSPHERE (section 18.2)
18.21 Do the reactions involved in ozone depletion involve changes in oxidation state of the O atoms? Explain.
18.22 Explain how the reactions of ozone in the stratosphere are responsible for the relatively warm temperatures of the stratosphere.
______
18.23 (a) What is the difference between chlorofluorocarbons and hydrofluorocarbons? (b) Why are hydrofluorocarbons potentially less harmful to the ozone layer than CFCs?
18.24 Draw the Lewis structure for the chlorofluorocarbon CFC-11, CFCl3. What chemical characteristics of this substance allow it to effectively deplete stratospheric ozone?
______
18.25 (a) Why is the fluorine present in chlorofluorocarbons not a major contributor to depletion of the ozone layer? (b) What are the chemical forms in which chlorine exists in the stratosphere following cleavage of the carbon–chlorine bond?
18.26 Would you expect the substance CFBr3 to be effective in depleting the ozone layer, assuming that it is present in the stratosphere? Explain.
______
18.27 For each of the following gases, make a list of known or possible naturally occurring sources: (a) CH4, (b) SO2, (c) NO.
18.28 Why is rainwater naturally acidic, even in the absence of polluting gases such as SO2?
______
18.29 (a) Write a chemical equation that describes the attack of acid rain on limestone, CaCO3. (b) If a limestone sculpture were treated to form a surface layer of calcium sulfate, would this help to slow down the effects of acid rain? Explain.
18.30 The first stage in corrosion of iron upon exposure to air is oxidation to Fe2+. (a) Write a balanced chemical equation to show the reaction of iron with oxygen and protons from acid rain. (b) Would you expect the same sort of reaction to occur with a silver surface? Explain.
______
18.31 Alcohol-based fuels for automobiles lead to the production of formaldehyde (CH2O) in exhaust gases. Formaldehyde undergoes photodissociation, which contributes to photochemical smog:
![]()
The maximum wavelength of light that can cause this reaction is 335 nm. (a) In what part of the electromagnetic spectrum is light with this wavelength found? (b) What is the maximum strength of a bond, in kJ/mol, that can be broken by absorption of a photon of 335-nm light? (c)Compare your answer from part (b) to the appropriate value from Table 8.4. What do you conclude about C—H bond energy in formaldehyde? (d) Write out the formaldehyde photodissociation reaction, showing Lewis-dot structures.
18.32 An important reaction in the formation of photochemical smog is the photodissociation of NO2:
![]()
The maximum wavelength of light that can cause this reaction is 420 nm. (a) In what part of the electromagnetic spectrum is light with this wavelength found? (b) What is the maximum strength of a bond, in kJ/mol, that can be broken by absorption of a photon of 420-nm light? (c)Write out the photodissociation reaction showing Lewis-dot structures.
______
18.33 Explain why increasing concentrations of CO2 in the atmosphere affect the quantity of energy leaving Earth but do not affect the quantity of energy entering from the Sun.
18.34 (a) With respect to absorption of radiant energy, what distinguishes a greenhouse gas from a nongreenhouse gas? (b) CH4 is a greenhouse gas, but N2 is not. How might the molecular structure of CH4 explain why it is a greenhouse gas?
EARTH'S WATER (section 18.3)
18.35 What is the molarity of Na+ in a solution of NaCl whose salinity is 5.6 if the solution has a density of 1.03 g/mol?
18.36 Phosphorus is present in seawater to the extent of 0.07 ppm by mass. If the phosphorus is present as phosphate, PO43–, calculate the corresponding molar concentration of phosphate in seawater.
______
18.37 The enthalpy of evaporation of water is 40.67 kJ/mol. Sunlight striking Earth's surface supplies 168 W per square meter (1 W = 1 watt = 1 J/s). (a) Assuming that evaporation of water is only due to energy input from the Sun, calculate how many grams of water could be evaporated from a 1.00 square meter patch of ocean over a 12-hour day. (b) The specific heat capacity of liquid water is 4.184 J/g °C. If the initial temperature of a 1.00 square meter patch of ocean is 26 °C, what is its final temperature after being in sunlight for 12 hours, assuming no phase changes and assuming that sunlight penetrates uniformly to depth of 10.0 cm?
[18.38] The enthalpy of fusion of water is 6.01 kJ/mol. Sunlight striking Earth's surface supplies 168 W per square meter (1 W = 1 watt = 1 J/s). (a) Assuming that melting of ice is only due to energy input from the Sun, calculate how many grams of ice could be melted from a 1.00 square meter patch of ice over a 12hour day. (b) The specific heat capacity of ice is 2.032 J/g °C. If the initial temperature of a 1.00 square meter patch of ice is –5.0 °C, what is its final temperature after being in sunlight for 12 hours, assuming no phase changes and assuming that sunlight penetrates uniformly to a depth of 1.00 cm?
______
18.39 A first-stage recovery of magnesium from seawater is precipi tation of Mg(OH)2 with CaO:
![]()
What mass of CaO, in grams, is needed to precipitate 1000 lb of Mg(OH)2?
18.40 Gold is found in seawater at very low levels, about 0.05 ppb by mass. Assuming that gold is worth about $800 per troy ounce, how many liters of seawater would you have to process to obtain $1,000,000 worth of gold? Assume the density of seawater is 1.03 g/mL and that your gold recovery process is 50% efficient.
______
18.41 (a) What is groundwater? (b) What is an aquijer?
18.42 The Ogallala aquifer is the largest in the United States, covering 450,000 km2 across eight states, from South Dakota to Texas. This aquifer provides 82% of the drinking water for the people who live in this region, although most (>75%) of the water that is pumped from it is for irrigation. Irrigation withdrawals are approximately 18 billion gallons per day. (a) The Ogallala aquifer might run dry, according to some estimates, in 25 years. How many cubic kilometers of water would be withdrawn in a 25-year period? (b) Explain the processes that would recharge the aquifer.
HUMAN ACTIVITIES AND EARTH'S WATER (section 18.4)
18.43 Suppose that one wishes to use reverse osmosis to reduce the salt content of brackish water containing 0.22 M total salt concentration to a value of 0.01 M, thus rendering it usable for human consumption. What is the minimum pressure that needs to be applied in the permeators (Figure 18.19) to achieve this goal, assuming that the operation occurs at 298 K? (Hint: Refer to Section 13.5.)
18.44 Assume that a portable reverse-osmosis apparatus operates on seawater, whose concentrations of constituent ions are listed in Table 18.5, and that the desalinated water output has an effective molarity of about 0.02 M. What minimum pressure must be applied by hand pumping at 297 K to cause reverse osmosis to occur? (Hint: Refer to Section 13.5.)
______
18.45 List the common products formed when an organic material containing the elements carbon, hydrogen, oxygen, sulfur, and nitrogen decomposes (a) under aerobic conditions, (b) under anaerobic conditions.
18.46 (a) Explain why the concentration of dissolved oxygen in freshwater is an important indicator of the quality of the water. (b) How is the solubility of oxygen in water affected by increasing temperature?
______
18.47 The organic anion
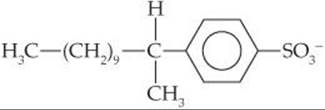
is found in most detergents. Assume that the anion undergoes aerobic decomposition in the following manner:
![]()
What is the total mass of O2 required to biodegrade 10.0 g of this substance?
18.48 The average daily mass of O2 taken up by sewage discharged in the United States is 59 g per person. How many liters of water at 9 ppm O2 are totally depleted of oxygen in 1 day by a population of 1,200,000 people?
______
18.49 Write a balanced chemical equation to describe how magnesium ions are removed in water treatment by the addition of slaked lime, Ca(OH)2.
18.50 (a) Which of the following ionic species could be responsible for hardness in a water supply: Ca2+, K+, Mg2+, Fe2+, Na+? (b) What properties of an ion determine whether it will contribute to water hardness?
______
18.51 How many moles of Ca(OH)2 and Na2CO3 should be added to soften 1200 L of water in which [Ca2+] = 5.0 × 10–4M and [HCO3–] = 7.0 × 10–4M?
18.52 The concentration of Ca2+ in a particular water supply is 5.7 × 10–3M. The concentration of bicarbonate ion, HCO3–, in the same water is 1.7 × 10–3M. What masses of Ca(OH)2 and Na2CO3 must be added to 5.0 × 107 L of this water to reduce the level of Ca2+ to 20% of its original level?
______
18.53 Ferrous sulfate (FeSO4) is often used as a coagulant in water purification. The iron(II) salt is dissolved in the water to be purified, then oxidized to the iron(III) state by dissolved oxygen, at which time gelatinous Fe(OH)3 forms, assuming the pH is above approximately 6. Write balanced chemical equations for the oxidation of Fe2+ to Fe3+ by dissolved oxygen and for the formation of Fe(OH)3(s) by reaction of Fe3+(aq) with HCO3–(aq).
18.54 What properties make a substance a good coagulant for water purification?
______
18.55 (a) What are trihalomethanes (THMs)? (b) Draw the Lewis structures of two example THMs.
18.56 If trihalomethanes are easily removed from water by aeration (bubbling with air), what does this imply about the vapor pressure of THMs compared to water?
GREEN CHEMISTRY (section 18.5)
18.57 One of the principles of green chemistry is that it is better to use as few steps as possible in making new chemicals. How does this principle relate to energy efficiency?
18.58 Discuss how catalysts can make processes more energy efficient.
______
18.59 A reaction for converting ketones to lactones, called the Baeyer–Villiger reaction,
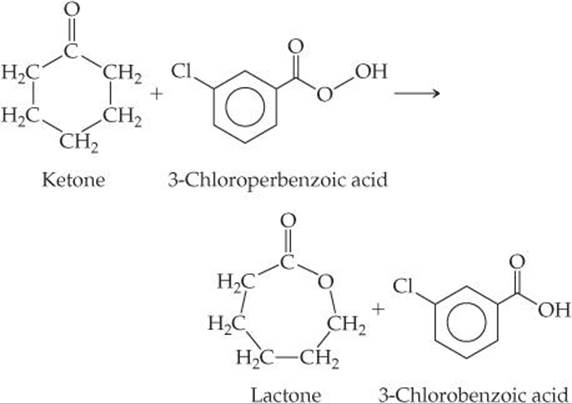
is used in the manufacture of plastics and pharmaceuticals. 3-Chloroperbenzoic acid is shock-sensitive, however, and prone to explode. Also, 3-chlorobenzoic acid is a waste product. An alternative process being developed uses hydrogen peroxide and a catalyst consisting of tin deposited within a solid support. The catalyst is readily recovered from the reaction mixture. (a) What would you expect to be the other product of oxidation of the ke-tone to lactone by hydrogen peroxide? (b) What principles of green chemistry are addressed by use of the proposed process?
18.60 The reaction shown here was performed with an iridium catalyst, both in supercritical CO2 (scCO2) and in the chlorinated solvent CH2Cl2. The kinetic data for the reaction in both solvents are plotted in the graph. Why is this a good example of a green chemical reaction?
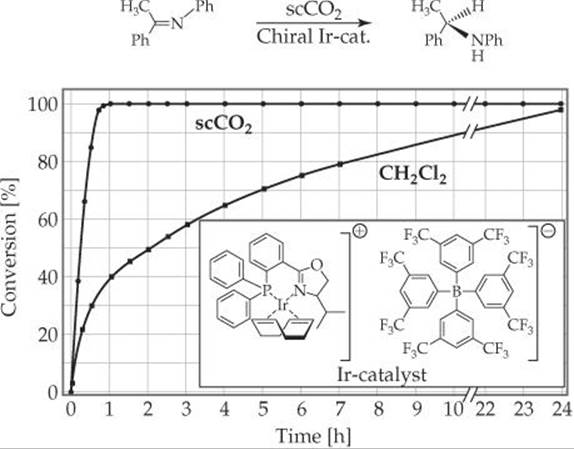
______
18.61 Which choice is greener in a chemical process? Explain. (a) Benzene as a solvent or water as a solvent. (b) The reaction temperature is 500 K, or 1000 K. (c) Sodium chloride as a by-product or chloroform (CHCl3) as a by-product.
18.62 Which choice is greener in a chemical process? Explain. (a) A reaction that can be run at 350 K for 12 hours without a catalyst or one that can be run at 300 K for 1 hour with a catalyst. (b) A reagent for the reaction that can be obtained from corn husks or one that can be obtained from petroleum. (c) A process that produces no by-products or one in which the byproducts are recycled for another process.
ADDITIONAL EXERCISES
18.63 A friend of yours has seen each of the following items in newspaper articles and would like an explanation: (a) acid rain, (b) greenhouse gas, (c) photochemical smog, (d) ozone depletion. Give a brief explanation of each term and identify one or two of the chemicals associated with each.
18.64 Suppose that on another planet the atmosphere consists of 17% Kr, 38% CH4, and 45% O2. What is the average molar mass at the surface? What is the average molar mass at an altitude at which all the O2 is photodissociated?
18.65 If an average O3 molecule “lives” only 100–200 seconds in the stratosphere before undergoing dissociation, how can O3 offer any protection from ultraviolet radiation?
18.66 Show how Equations 18.7 and 18.9 can be added to give Equation 18.10.
18.67 What properties of CFCs make them ideal for various commercial applications but also make them a long-term problem in the stratosphere?
18.68 Halons are fluorocarbons that contain bromine, such as CBrF3. They are used extensively as foaming agents for fighting fires. Like CFCs, halons are very unreactive and ultimately can diffuse into the stratosphere. (a) Based on the data in Table 8.4, would you expect photodissociation of Br atoms to occur in the stratosphere? (b) Propose a mechanism by which the presence of halons in the stratosphere could lead to the depletion of stratospheric ozone.
18.69 It is estimated that the lifetime for HFCs in the stratosphere is 2–7 years. If HFCs have such long lifetimes, why are they being used to replace CFCs?
[18.70] The hydroxyl radical, OH, is formed at low altitudes via the reaction of excited oxygen atoms with water:
![]()
(a) Write the Lewis structure for the hydroxyl radical. (Hint: It has one unpaired electron.)
Once produced, the hydroxyl radical is very reactive. Explain why each of the following series of reactions affects the pollution in the troposphere:
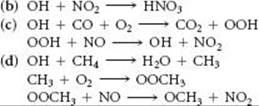
(e) The concentration of hydroxyl radicals in the troposphere is approximately 2 × 106 radicals per cm3. This estimate is based on a method called long path absorption spectroscopy (LPAS), similar in principle to the Beer's law measurement discussed in the Closer Look essay on p. 564, except that the path length in the LPAS measurement is 20 km. Why must the path length be so large?
18.71 Explain, using Le Châtelier's principle, why the equilibrium constant for the formation of NO from N2 and O2 increases with increasing temperature, whereas the equilibrium constant for the formation of NO2 from NO and O2 decreases with increasing temperature.
18.72 Natural gas consists primarily of methane, CH4(g). (a) Write a balanced chemical equation for the complete combustion of methane to produce CO2(g) as the only carbon-containing product. (b) Write a balanced chemical equation for the incomplete combustion of methane to produce CO(g) as the only carbon-containing product. (c) At 25 °C and 1.0 atm pressure, what is the minimum quantity of dry air needed to combust 1.0 L of CH4(g) completely to CO2(g)?
18.73 One of the possible consequences of climate change is an increase in the temperature of ocean water. The oceans serve as a “sink” for CO2 by dissolving large amounts of it. (a) How would the solubility of CO2 in the oceans be affected by an increase in the temperature of the water? (b) Discuss the implications of your answer to part (a) for the problem of climate change.
18.74 The rate of solar energy striking Earth averages 168 watts per square meter. The rate of energy radiated from Earth's surface averages 390 watts per square meter. Comparing these numbers, one might expect that the planet would cool quickly, yet it does not. Why not?
18.75 The solar power striking Earth every day averages 168 watts per square meter. The peak electrical power usage in New York City is 12,000 megawatts. Considering that present technology for solar energy conversion is only about 10% efficient, from how many square meters of land must sunlight be collected in order to provide this peak power? (For comparison, the total area of the city is 830 km2.)
18.76 Write balanced chemical equations for each of the following reactions: (a) The nitric oxide molecule undergoes photodis-sociation in the upper atmosphere. (b) The nitric oxide molecule undergoes photoionization in the upper atmosphere. (c) Nitric oxide undergoes oxidation by ozone in the stratosphere. (d) Nitrogen dioxide dissolves in water to form nitric acid and nitric oxide.
18.77 (a) Explain why Mg(OH)2 precipitates when CO32– ion is added to a solution containing Mg2+. (b) Will Mg(OH)2 precipitate when 4.0 g of Na2CO3 is added to 1.00 L of a solution containing 125 ppm of Mg2+?
[18.78] It has been pointed out that there may be increased amounts of NO in the troposphere as compared with the past because of massive use of nitrogen-containing compounds in fertilizers. Assuming that NO can eventually diffuse into the stratosphere, how might it affect the conditions of life on Earth? Using the index to this text, look up the chemistry of nitrogen oxides. What chemical pathways might NO in the troposphere follow?
[18.79] As of the writing of this text, EPA standards limit atmospheric ozone levels in urban environments to 84 ppb. How many moles of ozone would there be in the air above Los Angeles County (area about 4000 square miles; consider a height of 10 m above the ground) if ozone was at this concentration?
INTEGRATIVE EXERCISES
18.80 The estimated average concentration of NO2 in air in the United States in 2006 was 0.016 ppm. (a) Calculate the partial pressure of the NO2 in a sample of this air when the atmospheric pressure is 755 torr (99.1 kPa). (b) How many molecules of NO2 are present under these conditions at 20 °C in a room that measures 15 × 14 × 8 ft?
[18.81] In 1986 an electrical power plant in Taylorsville, Georgia, burned 8,376,726 tons of coal, a national record at that time. (a) Assuming that the coal was 83% carbon and 2.5% sulfur and that combustion was complete, calculate the number of tons of carbon dioxide and sulfur dioxide produced by the plant during the year. (b) If 55% of the SO2 could be removed by reaction with powdered CaO to form CaSO3, how many tons of CaSO3 would be produced?
18.82 The water supply for a midwestern city contains the following impurities: coarse sand, finely divided particulates, nitrate ion, trihalomethanes, dissolved phosphorus in the form of phosphates, potentially harmful bacterial strains, dissolved organic substances. Which of the following processes or agents, if any, is effective in removing each of these impurities: coarse sand filtration, activated carbon filtration, aeration, ozonization, precipitation with aluminum hydroxide?
18.83 An impurity in water has an extinction coefficient of 3.45 × 103M–1 cm–1 at 280 nm, its absorption maximum (A Closer Look, p. 564). Below 50 ppb, the impurity is not a problem for human health. Given that most spectrometers cannot detect absorbances less than 0.0001 with good reliability, is measuring the absorbance of water at 280 nm a good way to detect concentrations of the impurity above the 50-ppb threshold?
18.84 The concentration of H2O in the stratosphere is about 5 ppm. It undergoes photodissociation according to:
![]()
(a) Write out the Lewis-dot structures for both products and reactant.
(b) Using Table 8.4, calculate the wavelength required to cause this dissociation.
(c) The hydroxyl radicals, OH, can react with ozone, giving the following reactions:
![]()
What overall reaction results from these two elementary reactions? What is the catalyst in the overall reaction? Explain.
18.85 Bioremediation is the process by which bacteria repair their environment in response, for example, to an oil spill. The efficiency of bacteria for “eating” hydrocarbons depends on the amount of oxygen in the system, pH, temperature, and many other factors. In a certain oil spill, hydrocarbons from the oil disappeared with a first-order rate constant of 2 × 10–6 s–1. How many days did it take for the hydrocarbons to decrease to 10% of their initial value?
18.86 The standard enthalpies of formation of ClO and ClO2 are 101 and 102 kJ/mol, respectively. Using these data and the thermodynamic data in Appendix C, calculate the overall enthalpy change for each step in the following catalytic cycle:
![]()
What is the enthalpy change for the overall reaction that results from these two steps?
18.87 The main reason that distillation is a costly method for purifying water is the high energy required to heat and vaporize water. (a) Using the density, specific heat, and heat of vaporization of water from Appendix B, calculate the amount of energy required to vaporize 1.00 gal of water beginning with water at 20 °C. (b) If the energy is provided by electricity costing $0.085/kWh, calculate its cost. (c) If distilled water sells in a grocery store for $1.26 per gal, what percentage of the sales price is represented by the cost of the energy?
[18.88] A reaction that contributes to the depletion of ozone in the stratosphere is the direct reaction of oxygen atoms with ozone:
![]()
At 298 K the rate constant for this reaction is 4.8 ×105M–1s–1. (a) Based on the units of the rate constant, write the likely rate law for this reaction. (b) Would you expect this reaction to occur via a single elementary process? Explain why or why not. (c) From the magnitude of the rate constant, would you expect the activation energy of this reaction to be large or small? Explain. (d) Use ![]() values from Appendix C to estimate the enthalpy change for this reaction. Would this reaction raise or lower the temperature of the stratosphere?
values from Appendix C to estimate the enthalpy change for this reaction. Would this reaction raise or lower the temperature of the stratosphere?
18.89 Nitrogen dioxide (NO2) is the only important gaseous species in the lower atmosphere that absorbs visible light. (a) Write the Lewis structure(s) for NO2. (b) How does this structure account for the fact that NO2 dimerizes to form N2O4? Based on what you can find about this dimerization reaction in the text, would you expect to find the NO2 that forms in an urban environment to be in the form of dimer? Explain. (c) What would you expect as products, if any, for the reaction of NO2 with CO? (d) Would you expect NO2 generated in an urban environment to migrate to the stratosphere? Explain.
18.90 The following data were collected for the destruction of O3 by ![]() at very low concentrations:
at very low concentrations:
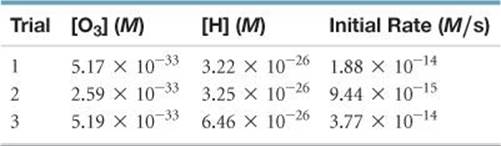
(a) Write the rate law for the reaction.
(b) Calculate the rate constant.
18.91 The degradation of CF3CH2F (an HFC) by OH radicals in the troposphere is first order in each reactant and has a rate constant of k = 1.6 × 108M –1s–1 at 4 °C. If the tropo-spheric concentrations of OH and CF3CH2F are 8.1 × 105 and 6.3× 108 molecules/cm3, respectively, what is the rate of reaction at this temperature in M/s?
[18.92] The Henry's law constant for CO2 in water at 25 °C is 3.1 × 10–2M atm–1. (a) What is the solubility of CO2 in water at this temperature if the solution is in contact with air at normal atmospheric pressure? (b) Assume that all of this CO2 is in the form of H2CO3 produced by the reaction between CO2 and H2O:
![]()
What is the pH of this solution?
[18.93] If the pH of a 1.0-in. rainfall over 1500 mi2 is 3.5, how many kilograms of H2SO4 are present, assuming that it is the only acid contributing to the pH?
18.94 The precipitation of Al(OH)3 (Ksp = 1.3 × 10–33) is sometimes used to purify water. (a) Estimate the pH at which precipitation of Al(OH)3 will begin if 5.0 lb of Al2(SO4)3 is added to 2000 gal of water. (b) Approximately how many pounds of CaO must be added to the water to achieve this pH?
18.95 The valuable polymer polyurethane is made by a condensation reaction of alcohols (ROH) with compounds that contain an isocyanate group (RNCO). Two reactions that can generate a urethane monomer are shown here:
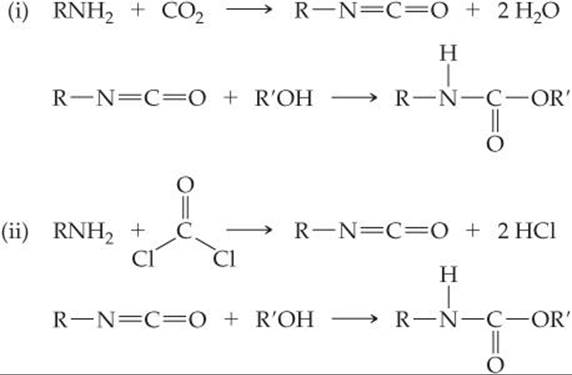
(a) Which process, i or ii, is greener? Explain.
(b) What are the hybridization and geometry of the carbon atoms in each C-containing compound in each reaction?
(c) If you wanted to promote the formation of the isocyanate intermediate in each reaction, what could you do, using Le Châtelier's principle?
[18.96] The pH of a particular raindrop is 5.6. (a) Assuming the major species in the raindrop are H2CO3(aq), HCO3–(aq), and CO32–(aq), calculate the concentrations of these species in the raindrop, assuming the total carbonate concentration is 1.0 × 10–5M. The appropriate Ka values are given in Table 16.3. (b) What experiments could you do to test the hypothesis that the rain also contains sulfur-containing species that contribute to its pH? Assume you have a large sample of rain to test.