CHEMISTRY THE CENTRAL SCIENCE
19 CHEMICAL THERMODYNAMICS
CHAPTER SUMMARY AND KEY TERMS
SECTION 19.1 Most reactions and chemical processes have an inherent directionality: They are spontaneous in one direction and nonspontaneous in the reverse direction. The spontaneity of a process is related to the thermodynamic path the system takes from the initial state to the final state. In a reversible process, both the system and its surroundings can be restored to their original state by exactly reversing the change. In an irreversible process the system cannot return to its original state without there being a permanent change in the surroundings. Any spontaneous process is irreversible. A process that occurs at a constant temperature is said to be isothermal.
SECTION 19.2 The spontaneous nature of processes is related to a thermodynamic state function called entropy, denoted S. For a process that occurs at constant temperature, the entropy change of the system is given by the heat absorbed by the system along a reversible path, divided by the temperature: ΔS = qrev/T. The way entropy controls the spontaneity of processes is given by the second law of thermodynamics, which governs the change in the entropy of the universe, ΔSuniv = ΔSsys + ΔSsurr. The second law states that in a reversible process ΔSuniv = 0; in an irreversible (spontaneous) process ΔSuniv > 0. Entropy values are usually expressed in units of joules per kelvin, J/K.
SECTION 19.3 A particular combination of motions and locations of the atoms and molecules of a system at a particular instant is called a microstate. The entropy of a system is a measure of its randomness or disorder. The entropy is related to the number of microstates, W, corresponding to the state of the system: S = k ln W. Molecules can undergo three kinds of motion: In translational motion the entire molecule moves in space. Molecules can also undergo vibrational motion, in which the atoms of the molecule move toward and away from one another in periodic fashion, and rotational motion, in which the entire molecule spins like a top. The number of available microstates, and therefore the entropy, increases with an increase in volume, temperature, or motion of molecules because any of these changes increases the possible motions and locations of the molecules. As a result, entropy generally increases when liquids or solutions are formed from solids, gases are formed from either solids or liquids, or the number of molecules of gas increases during a chemical reaction. The third law of thermodynamics states that the entropy of a pure crystalline solid at 0 K is zero.
SECTION 19.4 The third law allows us to assign entropy values for substances at different temperatures. Under standard conditions the entropy of a mole of a substance is called its standard molar entropy, denoted S°. From tabulated values of S°, we can calculate the entropy change for any process under standard conditions. For an isothermal process, the entropy change in the surroundings is equal to –ΔH/T.
SECTION 19.5 The Gibbs free energy (or just free energy), G, is a thermodynamic state function that combines the two state functions enthalpy and entropy: G = H – TS. For processes that occur at constant temperature, ΔG = ΔH – TΔS. For a process occurring at constant temperature and pressure, the sign of ΔG relates to the spontaneity of the process. When ΔG is negative, the process is spontaneous. When ΔG is positive, the process is nonspontaneous but the reverse process is spontaneous. At equilibrium the process is reversible and ΔG is zero. The free energy is also a measure of the maximum useful work that can be performed by a system in a spontaneous process. The standard free-energy change, ΔG°, for any process can be calculated from tabulations of standard free energies of formation, ![]() , which are defined in a fashion analogous to standard enthalpies of formation,
, which are defined in a fashion analogous to standard enthalpies of formation, ![]() . The value of
. The value of ![]() for a pure element in its standard state is defined to be zero.
for a pure element in its standard state is defined to be zero.
SECTIONS 19.6 AND 19.7 The values of ΔH and ΔS generally do not vary much with temperature. Therefore, the dependence of ΔG with temperature is governed mainly by the value of T in the expression ΔG = ΔH – TΔS. The entropy term –TΔS has the greater effect on the temperature dependence of ΔG and, hence, on the spontaneity of the process. For example, a process for which ΔH > 0 and ΔS > 0, such as the melting of ice, can be nonspontaneous (ΔG > 0) at low temperatures and spontaneous (ΔG < 0) at higher temperatures. Under nonstandard conditions ΔG is related to ΔG° and the value of the reaction quotient, Q: ΔG = ΔG° + RT ln Q. At equilibrium (ΔG = 0, Q = K), ΔG° = –RT ln K. Thus, the standard free-energy change is directly related to the equilibrium constant for the reaction. This relationship expresses the temperature dependence of equilibrium constants.
KEY SKILLS
• Understand the meaning of spontaneous process, reversible process, irreversible process, and isothermal process. (Section 19.1)
• State the second law of thermodynamics. (Section 19.2)
• Explain how the entropy of a system is related to the number of possible microstates. (Section 19.3)
• Describe the kinds of molecular motion that a molecule can possess. (Section 19.3)
• Predict the sign of ΔS for physical and chemical processes. (Section 19.3)
• State the third law of thermodynamics. (Section 19.3)
• Calculate standard entropy changes for a system from standard molar entropies. (Section 19.4)
• Calculate entropy changes in the surroundings for isothermal processes. (Section 19.4)
• Calculate the Gibbs free energy from the enthalpy change and entropy change at a given temperature. (Section 19.5)
• Use free-energy changes to predict whether reactions are spontaneous. (Section 19.5)
• Calculate standard free-energy changes using standard free energies of formation. (Section 19.5)
• Predict the effect of temperature on spontaneity given ΔH and ΔS. (Section 19.6)
• Calculate ΔG under nonstandard conditions. (Section 19.7)
• Relate ΔG° and equilibrium constant. (Section 19.7)
KEY EQUATIONS
![]()
Relating entropy change to the heat absorbed or released in a reversible process
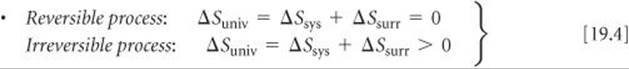
The second law of thermodynamics
![]()
Relating entropy to the number of microstates
![]()
Calculating the standard entropy change from standard molar entropies

The entropy change of the surroundings for a process at constant temperature and pressure
![]()
Calculating the Gibbs free-energy change from enthalpy and entropy changes at constant temperature
![]()
Calculating the standard free-energy change from standard free energies of formation
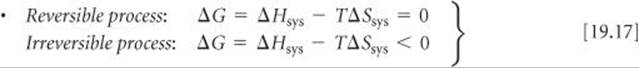
Relating the free-energy change to the reversibility of a process at constant temperature and pressure
![]()
Relating the free-energy change to the maximum work a process can perform
![]()
Calculating free-energy change under nonstandard conditions
![]()
Relating the standard free-energy change and the equilibrium constant