CHEMISTRY THE CENTRAL SCIENCE
20 ELECTRO-CHEMISTRY
20.5 FREE ENERGY AND REDOX REACTIONS
We have observed that voltaic cells use spontaneous redox reactions to produce a positive cell potential. We can use this fact together with half-cell potentials to decide whether a given redox reaction is spontaneous. In doing so, it is useful to make Equation 20.8 more general so that we can see how it pertains to general redox reactions, not just reactions in voltaic cells:
![]()
In writing the equation this way, we have dropped the subscript “cell” to indicate that the calculated emf does not necessarily refer to a voltaic cell. Also, we have generalized the standard reduction potentials by using the general terms reduction and oxidation rather than the terms specific to voltaic cells, cathode and anode. We can now make a general statement about the spontaneity of a reaction and its associated emf, E: A positive value of E indicates a spontaneous process; a negative value of E indicates a nonspontaneous process. We use E to represent the emf under nonstandard conditions and E° to indicate the standard emf.
SAMPLE EXERCISE 20.9 Determining Spontaneity
Use Table 20.1 to determine whether the following reactions are spontaneous under standard conditions.
![]()
SOLUTION
Analyze We are given two reactions and must determine whether each is spontaneous.
Plan To determine whether a redox reaction is spontaneous under standard conditions, we first need to write its reduction and oxidation half-reactions. We can then use the standard reduction potentials and Equation 20.10 to calculate the standard emf, E°, for the reaction. If a reaction is spontaneous, its standard emf must be a positive number.
Solve
(a) For oxidation of Cu to Cu2+ and reduction of H+ to H2, the half-reactions and standard reduction potentials are

Notice that for the oxidation, we use the standard reduction potential from Table 20.1 for the reduction of Cu2+ to Cu. We now calculate E° by using Equation 20.10:
![]()
Because E° is negative, the reaction is not spontaneous in the direction written. Copper metal does not react with acids in this fashion. The reverse reaction, however, is spontaneous and has a positive E° value:
![]()
Thus, Cu2+ can be reduced by H2.
(b) We follow a procedure analogous to that in (a):

In this case
![]()
Because the value of E° is positive, this reaction is spontaneous and could be used to build a voltaic cell.
PRACTICE EXERCISE
Using the standard reduction potentials listed in Appendix E, determine which of the following reactions are spontaneous under standard conditions:
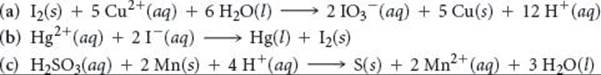
Answer: Reactions (b) and (c) are spontaneous.
We can use standard reduction potentials to understand the activity series of metals. ![]() (Section 4.4) Recall that any metal in the activity series (Table 4.5) is oxidized by the ions of any metal below it. We can now recognize the origin of this rule based on standard reduction potentials. The activity series is based on the oxidation reactions of the metals, ordered from strongest reducing agent at the top to weakest reducing agent at the bottom. (Thus, the ordering is inverted relative to that in Table 20.1.) For example, nickel lies above silver in the activity series, making nickel the stronger reducing agent. Because a reducing agent is oxidized in any redox reaction, nickel is more easily oxidized than silver. In a mixture of nickel metal and silver cations, therefore, we expect a displacement reaction in which the silver ions are displaced in the solution by nickel ions:
(Section 4.4) Recall that any metal in the activity series (Table 4.5) is oxidized by the ions of any metal below it. We can now recognize the origin of this rule based on standard reduction potentials. The activity series is based on the oxidation reactions of the metals, ordered from strongest reducing agent at the top to weakest reducing agent at the bottom. (Thus, the ordering is inverted relative to that in Table 20.1.) For example, nickel lies above silver in the activity series, making nickel the stronger reducing agent. Because a reducing agent is oxidized in any redox reaction, nickel is more easily oxidized than silver. In a mixture of nickel metal and silver cations, therefore, we expect a displacement reaction in which the silver ions are displaced in the solution by nickel ions:
![]()
In this reaction Ni is oxidized and Ag+ is reduced. Therefore, the standard emf for the reaction is
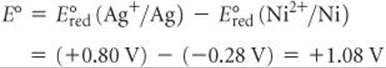
The positive value of E° indicates that the displacement of silver by nickel resulting from oxidation of Ni metal and reduction of Ag+ is a spontaneous process. Remember that although we multiply the silver half-reaction by 2, the reduction potential is not multiplied.
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
Based on Table 4.5, which is the stronger reducing agent, Hg(l) or Pb(s)?
Emf, Free Energy, and the Equilibrium Constant
The change in the Gibbs free energy, ΔG, is a measure of the spontaneity of a process that occurs at constant temperature and pressure. ![]() (Section 19.5) The emf, E, of a redox reaction also indicates whether the reaction is spontaneous. The relationship between emf and the free-energy change is
(Section 19.5) The emf, E, of a redox reaction also indicates whether the reaction is spontaneous. The relationship between emf and the free-energy change is
![]()
In this equation, n is a positive number without units that represents the number of moles of electrons transferred according to the balanced equation for the reaction, and F is Faraday's constant, named after Michael Faraday (![]() FIGURE 20.13):
FIGURE 20.13):
![]()

![]() FIGURE 20.13 Michael Faraday. Faraday (1791-1867) was born in England, a child of a poor blacksmith. At the age of 14 he was apprenticed to a bookbinder who gave him time to read and to attend lectures. In 1812 he became an assistant in Humphry Davy's laboratory at the Royal Institution. He succeeded Davy as the most famous and influential scientist in England, making an amazing number of important discoveries, including his formulation of the quantitative relationships between electrical current and the extent of chemical reaction in electrochemical cells.
FIGURE 20.13 Michael Faraday. Faraday (1791-1867) was born in England, a child of a poor blacksmith. At the age of 14 he was apprenticed to a bookbinder who gave him time to read and to attend lectures. In 1812 he became an assistant in Humphry Davy's laboratory at the Royal Institution. He succeeded Davy as the most famous and influential scientist in England, making an amazing number of important discoveries, including his formulation of the quantitative relationships between electrical current and the extent of chemical reaction in electrochemical cells.
Faraday's constant is the quantity of electrical charge on 1 mol of electrons.
The units of ΔG calculated with Equation 20.11 are J/mol. As in Equation 19.19, we use “per mole” to mean per mole of reaction as indicated by the coefficients in the balanced equation. ![]() (Section 19.7)
(Section 19.7)
Because both n and F are positive numbers, a positive value of E in Equation 20.11 leads to a negative value of ΔG. Remember: A positive value of E and a negative value of ΔG both indicate a spontaneous reaction. When the reactants and products are all in their standard states, Equation 20.11 can be modified to relate ΔG° and E°:
![]()
Because ΔG° is related to the equilibrium constant, K, for a reaction by the expression ΔG° = –RT ln K (Equation 19.20), we can relate E° to K by solving Equation 20.12 for E° and then substituting the Equation 19.20 expression for ΔG°:
![]()
![]() FIGURE 20.14 summarizes the relationships among E°, ΔG°, and K.
FIGURE 20.14 summarizes the relationships among E°, ΔG°, and K.
![]() GO FIGURE
GO FIGURE
What does the variable n represent in the ΔG° and E° equations?
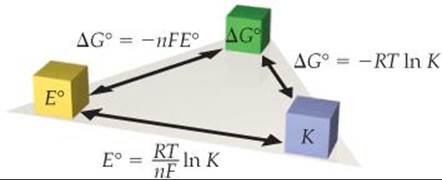
![]() FIGURE 20.14 Relationships of E°, ΔG°, and K. Any one of these important parameters can be used to calculate the other two. The signs of E° and ΔG° determine the direction that the reaction proceeds under standard conditions. The magnitude of K determines the relative amounts of reactants and products in an equilibrium mixture.
FIGURE 20.14 Relationships of E°, ΔG°, and K. Any one of these important parameters can be used to calculate the other two. The signs of E° and ΔG° determine the direction that the reaction proceeds under standard conditions. The magnitude of K determines the relative amounts of reactants and products in an equilibrium mixture.
SAMPLE EXERCISE 20.10 Using Standard Reduction Potentials to Calculate ΔG° andK
(a) Use the standard reduction potentials in Table 20.1 to calculate the standard free-energy change, ΔG°, and the equilibrium constant, K, at 298 K for the reaction
![]()
(b) Suppose the reaction in part (a) is written
![]()
What are the values of E°, ΔG°, and K when the reaction is written in this way?
SOLUTION
Analyze We are asked to determine ΔG° and K for a redox reaction, using standard reduction potentials.
Plan We use the data in Table 20.1 and Equation 20.10 to determine E° for the reaction and then use E° in Equation 20.12 to calculate ΔG°. We can then use Equation 19.20, ΔG° = –RT ln K, to calculate K.
Alternatively, we can calculate K using Equation 20.13, ![]() .
.
Solve
(a) We first calculate E° by breaking the equation into two half-reactions and obtaining ![]() values from Table 20.1 (or Appendix E):
values from Table 20.1 (or Appendix E):
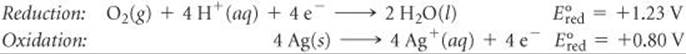
Even though the second half-reaction has 4 Ag, we use the ![]() value directly from Table 20.1 because emf is an intensive property.
value directly from Table 20.1 because emf is an intensive property.
Using Equation 20.10, we have
![]()
The half-reactions show the transfer of four electrons. Thus, for this reaction n = 4. We now use Equation 20.12 to calculate ΔG°:
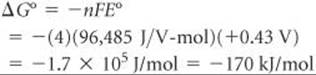
The positive value of E° leads to a negative value of ΔG°. The per mol part of the unit relates to the balanced equation, 4 Ag(s) + O2(g) + 4 H+(aq) → 4 Ag+(aq) + 2 H2O(l). Thus, –170 kJ is associated with 4 mol Ag, 1 mol O2 and 4 mol H+, and so forth, corresponding to the coefficients in the balanced equation.
Now we need to calculate the equilibrium constant, K, using ΔG° = RT ln K. Because ΔG° is a large negative number, which means the reaction is thermodynamically very favorable, we expect K to be large.
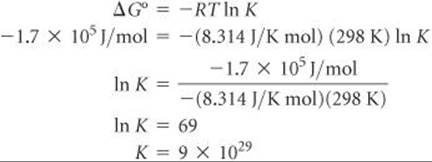
K is indeed very large! This means that we expect silver metal to oxidize in acidic aqueous environments, in air, to Ag+.
Notice that the emf calculated for the reaction was E° = 0.43 V, which is easy to measure. Directly measuring such a large equilibrium constant by measuring reactant and product concentrations at equilibrium, on the other hand, would be very difficult.
(b) The overall equation is the same as that in part (a), multiplied by ![]() . The half-reactions are
. The half-reactions are
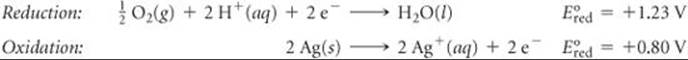
The values of ![]() are the same as they were in part (a); they are not changed by multiplying the half-reactions by
are the same as they were in part (a); they are not changed by multiplying the half-reactions by ![]() . Thus, E° has the same value as in part (a):
. Thus, E° has the same value as in part (a):
![]()
Notice, though, that the value of n has changed to n = 2, which is one-half the value in part (a). Thus, ΔG° is half as large as in part (a):
![]()
The value of ΔG° is half that in part (a) because the coefficients in the chemical equation are half those in (a).
Now we can calculate K as before:
![]()
Comment E° is an intensive quantity, so multiplying a chemical equation by a certain factor will not affect the value of E°. Multiplying an equation will change the value of n, however, and hence the value of ΔG°. The change in free energy, in units of J/mol of reaction as written, is anextensive quantity. The equilibrium constant is also an extensive quantity.
PRACTICE EXERCISE
For the reaction
![]()
(a) What is the value of n? (b) Use the data in Appendix E to calculate ΔG°. (c) Calculate K at T = 298 K.
Answers: (a) 6, (b) +87kJ/mol, (c) K = 6 × 10–16
 A CLOSER LOOK
A CLOSER LOOK
ELECTRICAL WORK
For any spontaneous process, ΔG is a measure of the maximum useful work, wmax, that can be extracted from the process: ΔG = wmax. ![]() (Section 19.5) Because ΔG = –nFE, the maximum useful electrical work obtainable from a voltaic cell is
(Section 19.5) Because ΔG = –nFE, the maximum useful electrical work obtainable from a voltaic cell is
![]()
Because cell emf, Ecell, is always positive for a voltaic cell, wmax is negative, indicating that work is done by a system on its surroundings, as we expect for a voltaic cell. ![]() (Section 5.2)
(Section 5.2)
As Equation 20.14 shows, the more charge a voltaic cell moves through a circuit (that is, the larger nF is) and the larger the emf pushing the electrons through the circuit (that is, the larger Ecell is), the more work the cell can accomplish. In Sample Exercise 20.10, we calculated ![]() for the reaction
for the reaction ![]() . Thus, a voltaic cell utilizing this reaction could perform a maximum of 170 kJ of work in consuming 4 mol Ag, 1 mol O2, and 4 mol H+.
. Thus, a voltaic cell utilizing this reaction could perform a maximum of 170 kJ of work in consuming 4 mol Ag, 1 mol O2, and 4 mol H+.
If a reaction is not spontaneous, ΔG is positive and E is negative. To force a nonspontaneous reaction to occur in an electrochemical cell, we need to apply an external potential, Eext, that exceeds ![]() . For example, if a nonspontaneous process has E = –0.9 V, then the external potentialEext must be greater than +0.9 V in order for the process to occur. We will examine such nonspontaneous processes in Section 20.9.
. For example, if a nonspontaneous process has E = –0.9 V, then the external potentialEext must be greater than +0.9 V in order for the process to occur. We will examine such nonspontaneous processes in Section 20.9.
Electrical work can be expressed in energy units of watts times time. The watt (W) is a unit of electrical power (that is, rate of energy expenditure):
![]()
Thus, a watt-second is a joule. The unit employed by electric utilities is the kilowatt-hour (kWh), which equals 3.6 × 106 J:
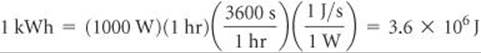
RELATED EXERCISES: 20.59, 20.60