CHEMISTRY THE CENTRAL SCIENCE
20 ELECTRO-CHEMISTRY
20.7 BATTERIES AND FUEL CELLS
A battery is a portable, self-contained electrochemical power source that consists of one or more voltaic cells. For example, the 1.5-V batteries used to power flashlights and many consumer electronic devices are single voltaic cells. Greater voltages can be achieved by using multiple cells, as in 12-V automotive batteries. When cells are connected in series (which means the cathode of one attached to the anode of another), the battery produces a voltage that is the sum of the voltages of the individual cells. Higher voltages can also be achieved by using multiple batteries in series (![]() FIGURE 20.18). Battery electrodes are marked following the convention of Figure 20.6—plus for cathode and minus for anode.
FIGURE 20.18). Battery electrodes are marked following the convention of Figure 20.6—plus for cathode and minus for anode.
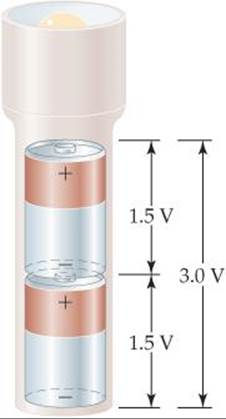
![]() FIGURE 20.18 Combining batteries. When batteries are connected in series, as in most flashlights, the total voltage is the sum of the individual voltages.
FIGURE 20.18 Combining batteries. When batteries are connected in series, as in most flashlights, the total voltage is the sum of the individual voltages.
Although any spontaneous redox reaction can serve as the basis for a voltaic cell, making a commercial battery that has specific performance characteristics can require considerable ingenuity. The substances oxidized at the anode and reduced by the cathode determine the voltage, and the usable life of the battery depends on the quantities of these substances packaged in the battery. Usually a barrier analogous to the porous barrier of Figure 20.6 separates the anode and cathode half-cells.
Different applications require batteries with different properties. The battery required to start a car, for example, must be capable of delivering a large electrical current for a short time period, whereas the battery that powers a heart pacemaker must be very small and capable of delivering a small but steady current over an extended time period. Some batteries are primary cells, meaning they cannot be recharged and must be either discarded or recycled after the voltage drops to zero. A secondary cell can be recharged from an external power source after its voltage has dropped.
As we consider some common batteries, notice how the principles we have discussed so far help us understand these important sources of portable electrical energy.
![]() GO FIGURE
GO FIGURE
What is the oxidation state of lead in the cathode of this battery?
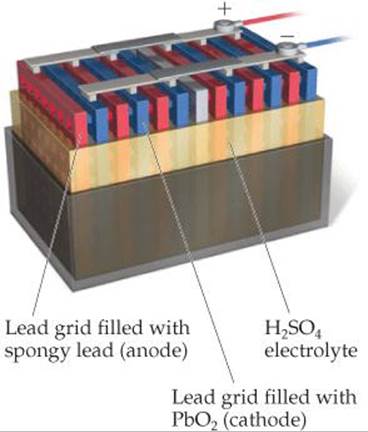
![]() FIGURE 20.19 A 12-V automotive lead-acid battery. Each anode/cathode pair in this schematic cutaway produces a voltage of about 2 V. Six pairs of electrodes are connected in series, producing 12 V.
FIGURE 20.19 A 12-V automotive lead-acid battery. Each anode/cathode pair in this schematic cutaway produces a voltage of about 2 V. Six pairs of electrodes are connected in series, producing 12 V.
Lead-Acid Battery
A 12-V lead-acid automotive battery consists of six voltaic cells in series, each producing 2 V. The cathode of each cell is lead dioxide (PbO2) packed on a lead grid (![]() FIGURE 20.19). The anode of each cell is lead. Both electrodes are immersed in sulfuric acid.
FIGURE 20.19). The anode of each cell is lead. Both electrodes are immersed in sulfuric acid.
The reactions that occur during discharge are
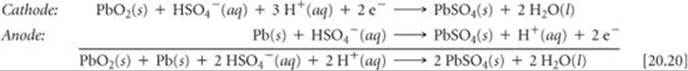
The standard cell potential can be obtained from the standard reduction potentials in Appendix E:
![]()
The reactants Pb and PbO2 are the electrodes. Because these reactants are solids, there is no need to separate the cell into half-cells; the Pb and PbO2 cannot come into contact with each other unless one electrode touches another. To keep the electrodes from touching, wood or glass-fiber spacers are placed between them (Figure 20.19). Using a reaction whose reactants and products are solids has another benefit. Because solids are excluded from the reaction quotient Q, the relative amounts of Pb(s), PbO2(s), and PbSO4(s) have no effect on the voltage of the lead storage battery, helping the battery maintain a relatively constant voltage during discharge. The voltage does vary somewhat with use because the concentration of H2SO4 varies with the extent of discharge. As Equation 20.20 indicates, H2SO4 is consumed during the discharge.
A major advantage of the lead-acid battery is that it can be recharged. During recharging, an external source of energy is used to reverse the direction of the cell reaction, regenerating Pb(s) and PbO2(s):
![]()
In an automobile the alternator provides the energy necessary for recharging the battery. Recharging is possible because PbSO4 formed during discharge adheres to the electrodes. As the external source forces electrons from one electrode to the other, the PbSO4 is converted to Pb at one electrode and to PbO2 at the other.
Alkaline Battery
The most common primary (nonrechargeable) battery is the alkaline battery (![]() FIGURE 20.20). The anode is powdered zinc metal immobilized in a gel in contact with a concentrated solution of KOH (hence, the name alkaline battery). The cathode is a mixture of MnO2(s) and graphite, separated from the anode by a porous fabric. The battery is sealed in a steel can to reduce the risk of any of the concentrated KOH escaping.
FIGURE 20.20). The anode is powdered zinc metal immobilized in a gel in contact with a concentrated solution of KOH (hence, the name alkaline battery). The cathode is a mixture of MnO2(s) and graphite, separated from the anode by a porous fabric. The battery is sealed in a steel can to reduce the risk of any of the concentrated KOH escaping.
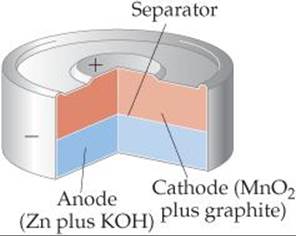
![]() FIGURE 20.20 Cutaway view of a miniature alkaline battery.
FIGURE 20.20 Cutaway view of a miniature alkaline battery.
The cell reactions are complex but can be approximately represented as follows:
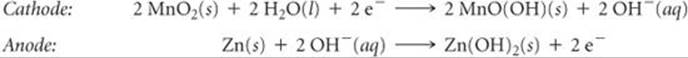
Nickel-Cadmium, Nickel-Metal-Hydride, and Lithium-Ion Batteries
The tremendous growth in high-power-demand portable electronic devices in the last decade has increased the demand for lightweight, readily recharged batteries. One of the most common rechargeable batteries is the nickel-cadmium (nicad) battery. During discharge, cadmium metal is oxidized at the anode while nickel oxyhydroxide [NiO(OH)(s)] is reduced at the cathode:
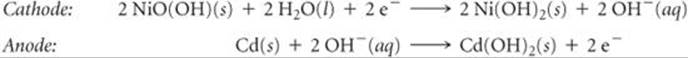
As in the lead-acid battery, the solid reaction products adhere to the electrodes, which permits the electrode reactions to be reversed during charging. A single nicad voltaic cell has a voltage of 1.30 V. Nicad battery packs typically contain three or more cells in series to produce the higher voltages needed by most electronic devices.
Two drawbacks to nickel-cadmium batteries are that cadmium is both toxic and dense. Its use increases battery weight and provides an environmental hazard—roughly 1.5 billion nickel-cadmium batteries are produced annually, and these must eventually be recycled as they lose their ability to be recharged. Some of the problem has been alleviated by the development of the nickel-metal-hydride (NiMH) battery. The cathode reaction is the same as that for nickel-cadmium batteries, but the anode reaction is very different. The anode consists of a metal alloy, such as ZrNi2, that has the ability to absorb hydrogen atoms. During oxidation at the anode, the hydrogen atoms lose electrons, and the resultant H+ ions react with OH– ions to form H2O, a process that is reversed during charging.
The current generation of hybrid gas–electric automobiles use NiMH batteries, which are recharged by the electric motor while braking. These batteries can last up to 8 years.
The newest rechargeable battery to receive large use in consumer devices is the lithium-ion (Li-ion) battery. You will find this battery in cell phones and laptop computers. Because lithium is a very light element, Li-ion batteries achieve a greater energy density—the amount of energy stored per unit mass—than nickel-based batteries. The technology of Li-ion batteries is based on the ability of Li+ ions to be inserted into and removed from certain layered solids. For example, Li+ ions can be inserted reversibly into layers of graphite (Figure 12.30). In most commercial cells, one electrode is graphite or some other carbon-based material, and the other is usually made of lithium cobalt oxide (LiCoO2). When the cell is charged, cobalt ions are oxidized and Li+ ions migrate into the graphite. During discharge, when the battery is producing electricity for use, the Li+ ions spontaneously migrate from the graphite anode to the cathode, enabling electrons to flow through the external circuit. An Li-ion battery produces a maximum voltage of 3.7 V, considerably higher than typical 1.5-V alkaline batteries.
Hydrogen Fuel Cells
The thermal energy released by burning fuels can be converted to electrical energy. The thermal energy may convert water to steam, for instance, which drives a turbine that in turn drives an electrical generator. Typically, a maximum of only 40% of the energy from combustion is converted to electricity in this manner; the remainder is lost as heat. The direct production of electricity from fuels by a voltaic cell could, in principle, yield a higher rate of conversion of chemical energy to electrical energy. Voltaic cells that perform this conversion using conventional fuels, such as H2 and CH4, are called fuel cells. Strictly speaking, fuel cells are not batteries because they are not self-contained systems—the fuel must be continuously supplied to generate electricity.
The most common fuel-cell systems involve the reaction of H2(g) and O2(g) to form H2O(l). These cells can generate electricity twice as efficiently as the best internal combustion engine. Under acidic conditions, the reactions are
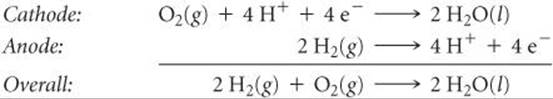
These cells employ hydrogen gas as the fuel and oxygen gas from air as the oxidant and generate about 1 V.
Fuel cells are often named for either the fuel or the electrolyte used. In the hydro-gen-PEM fuel cell (the acronym PEM stands for either proton-exchange membrane or polymer-electrolyte membrane), the anode and cathode are separated by a membrane, which is permeable to protons but not to electrons (![]() FIGURE 20.21). The membrane therefore acts as a salt bridge. The electrodes are typically made from graphite.
FIGURE 20.21). The membrane therefore acts as a salt bridge. The electrodes are typically made from graphite.
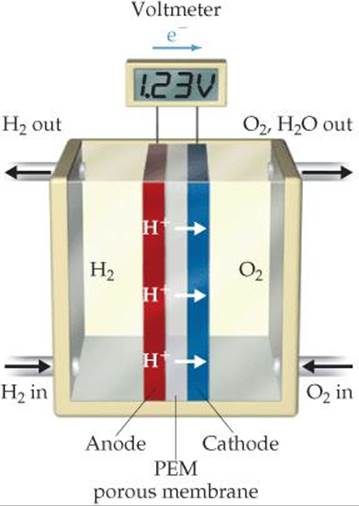
![]() FIGURE 20.21 A hydrogen-PEM fuel cell. The proton-exchange membrane (PEM) allows H+ ions generated by H2 oxidation at the anode to migrate to the cathode, where H2O is formed.
FIGURE 20.21 A hydrogen-PEM fuel cell. The proton-exchange membrane (PEM) allows H+ ions generated by H2 oxidation at the anode to migrate to the cathode, where H2O is formed.
The hydrogen-PEM cell operates at around 80°C. At this temperature the electrochemical reactions would normally occur very slowly, and so a thin layer of platinum on each electrode catalyzes the reactions. Many fuel cells require much higher temperatures to operate.
In order to power a vehicle, multiple cells must be assembled into a fuel cell stack. The amount of power generated by a stack depends on the number and size of the fuel cells in the stack and on the surface area of the PEM.
Much fuel cell research today is directed toward improving electrolytes and catalysts and toward developing cells that use fuels such as hydrocarbons and alcohols, which are not as difficult to handle and distribute as hydrogen gas.
 CHEMISTRY PUT TO WORK
CHEMISTRY PUT TO WORK
Direct Methanol Fuel Cells
Even though the hydrogen fuel cell is highly developed, liquid methanol, CH3OH, is a far more attractive fuel to store and transport than hydrogen gas. Furthermore, methanol is a clean-burning liquid, and its use would require only minor modifications to existing engines and to fuel-delivery infrastructure.
One of the intriguing aspects of methanol fuel is that manufacturing it could consume carbon dioxide, a source of global warming. ![]() (Section 18.2) Methanol can be made by combining CO2 and H2, although the process is presently costly. Imagine, though, that the synthesis can be improved and that the CO2 used in the synthesis is captured from exhaust gases from power plants or even directly from the atmosphere. In such cases, the CO2 released by subsequently burning the methanol would be canceled by the carbon dioxide captured to make it. Thus, the process would be carbon neutral, meaning that it would not increase the concentration of CO2 in the atmosphere. The prospect of a liquid fuel that could replace conventional fuels without contributing to the greenhouse effect has spurred considerable research to reduce the cost of methanol synthesis and to develop and improve methanol fuel cell technology.
(Section 18.2) Methanol can be made by combining CO2 and H2, although the process is presently costly. Imagine, though, that the synthesis can be improved and that the CO2 used in the synthesis is captured from exhaust gases from power plants or even directly from the atmosphere. In such cases, the CO2 released by subsequently burning the methanol would be canceled by the carbon dioxide captured to make it. Thus, the process would be carbon neutral, meaning that it would not increase the concentration of CO2 in the atmosphere. The prospect of a liquid fuel that could replace conventional fuels without contributing to the greenhouse effect has spurred considerable research to reduce the cost of methanol synthesis and to develop and improve methanol fuel cell technology.
A direct methanol fuel cell has been developed that is similar to the hydrogen-PEM fuel cell. The reactions in the cell are
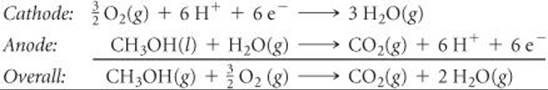
The direct methanol fuel cell is currently too expensive and too inefficient to be used in passenger cars. Nevertheless, small methanol fuel cells could appear in mobile devices such as computers or cell phones in the near future.