CHEMISTRY THE CENTRAL SCIENCE
20 ELECTRO-CHEMISTRY
20.8 CORROSION
In this section we examine the undesirable redox reactions that lead to corrosion of metals. Corrosion reactions are spontaneous redox reactions in which a metal is attacked by some substance in its environment and converted to an unwanted compound.
For nearly all metals, oxidation is thermodynamically favorable in air at room temperature. When oxidation of a metal object is not inhibited, it can destroy the object. Oxidation can form an insulating protective oxide layer, however, that prevents further reaction of the underlying metal. Based on the standard reduction potential for Al3+, for example, we expect aluminum metal to be readily oxidized. The many aluminum soft-drink and beer cans that litter the environment are ample evidence, however, that aluminum undergoes only very slow chemical corrosion. The exceptional stability of this active metal in air is due to the formation of a thin protective coat of oxide—a hydrated form of Al2O3—on the metal surface. The oxide coat is impermeable to O2 or H2O and so protects the underlying metal from further corrosion.
Magnesium metal is similarly protected, and some metal alloys, such as stainless steel, likewise form protective impervious oxide coats.
Corrosion of Iron (Rusting)
The rusting of iron is a familiar corrosion process that carries a significant economic impact. Up to 20% of the iron produced annually in the United States is used to replace iron objects that have been discarded because of rust damage.
Rusting of iron requires both oxygen and water, and the process can be accelerated by other factors such as pH, presence of salts, contact with metals more difficult to oxidize than iron, and stress on the iron. The corrosion process involves oxidation and reduction, and the metal conducts electricity. Thus, electrons can move through the metal from a region where oxidation occurs to a region where reduction occurs, as in voltaic cells. Because the standard reduction potential for reduction of Fe2+(aq) is less positive than that for reduction of O2, Fe(s) can be oxidized by O2(g):

A portion of the iron, often associated with a dent or region of strain, can serve as an anode at which Fe is oxidized to Fe2+ (![]() FIGURE 20.22). The electrons produced in the oxidation migrate through the metal from this anodic region to another portion of the surface, which serves as the cathode where O2 is reduced. The reduction of O2 requires H+, so lowering the concentration of H+ (increasing the pH) makes O2 reduction less favorable. Iron in contact with a solution whose pH is greater than 9 does not corrode.
FIGURE 20.22). The electrons produced in the oxidation migrate through the metal from this anodic region to another portion of the surface, which serves as the cathode where O2 is reduced. The reduction of O2 requires H+, so lowering the concentration of H+ (increasing the pH) makes O2 reduction less favorable. Iron in contact with a solution whose pH is greater than 9 does not corrode.
The Fe2+ formed at the anode is eventually oxidized to Fe3+, which forms the hydrated iron(III) oxide known as rust:*
![]()
![]() GO FIGURE
GO FIGURE
What is the oxidizing agent in this corrosion reaction?
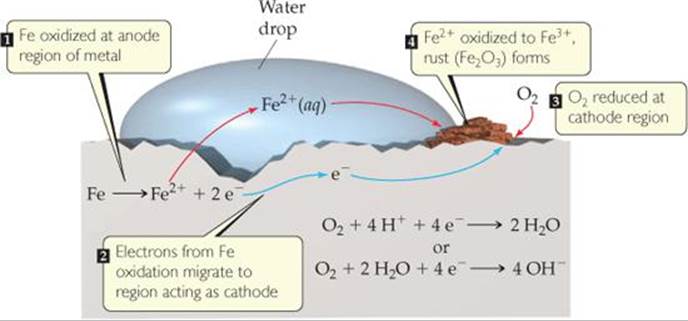
![]() FIGURE 20.22 Corrosion of iron in contact with water. One region of the iron acts as the cathode and another region acts as the anode.
FIGURE 20.22 Corrosion of iron in contact with water. One region of the iron acts as the cathode and another region acts as the anode.

![]() FIGURE 20.23 Cathodic protection of iron in contact with zinc. The standard reduction potentials are
FIGURE 20.23 Cathodic protection of iron in contact with zinc. The standard reduction potentials are ![]() , Fe2+ = –0.440 V,
, Fe2+ = –0.440 V, ![]() , Zn2+ = –0.763 V, making the zinc more readily oxidized.
, Zn2+ = –0.763 V, making the zinc more readily oxidized.
Because the cathode is generally the area having the largest supply of O2, rust often deposits there. If you look closely at a shovel after it has stood outside in the moist air with wet dirt adhered to its blade, you may notice that pitting has occurred under the dirt but that rust has formed elsewhere, where O2 is more readily available. The enhanced corrosion caused by the presence of salts is usually evident on autos in areas where roads are heavily salted during winter. Like a salt bridge in a voltaic cell, the ions of the salt provide the electrolyte necessary to complete the electrical circuit.
Preventing Corrosion of Iron
Objects made of iron are often covered with a coat of paint or another metal to protect against corrosion. Covering the surface with paint prevents oxygen and water from reaching the iron surface. If the coating is broken, however, and the iron exposed to oxygen and water, corrosion begins as the iron is oxidized.
With galvanized iron, which is iron coated with a thin layer of zinc, the iron is protected from corrosion even after the surface coat is broken. The standard reduction potentials are

Because ![]() for Fe2+ is less negative (more positive) than
for Fe2+ is less negative (more positive) than ![]() for Zn2+, Zn(s) is more readily oxidized than Fe(s). Thus, even if the zinc coating is broken and the galvanized iron is exposed to oxygen and water, as in
for Zn2+, Zn(s) is more readily oxidized than Fe(s). Thus, even if the zinc coating is broken and the galvanized iron is exposed to oxygen and water, as in ![]() FIGURE 20.23, the zinc serves as the anode and is corroded (oxidized) instead of the iron. The iron serves as the cathode at which O2 is reduced.
FIGURE 20.23, the zinc serves as the anode and is corroded (oxidized) instead of the iron. The iron serves as the cathode at which O2 is reduced.
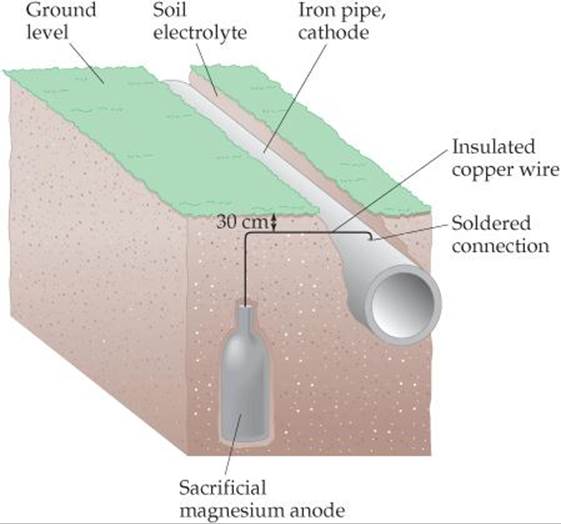
![]() FIGURE 20.24 Cathodic protection of an iron pipe. A mixture of gypsum, sodium sulfate, and clay surrounds the sacrificial magnesium anode to promote conductivity of ions.
FIGURE 20.24 Cathodic protection of an iron pipe. A mixture of gypsum, sodium sulfate, and clay surrounds the sacrificial magnesium anode to promote conductivity of ions.
Protecting a metal from corrosion by making it the cathode in an electrochemical cell is known as cathodic protection. The metal that is oxidized while protecting the cathode is called the sacrificial anode. Underground pipelines and storage tanks made of iron are often protected against corrosion by making the iron the cathode of a voltaic cell. For example, pieces of a metal that is more easily oxidized than iron, such as magnesium (![]() = –2.37 V), are buried near the pipe or storage tank and connected to it by wire (
= –2.37 V), are buried near the pipe or storage tank and connected to it by wire (![]() FIGURE 20.24). In moist soil, where corrosion can occur, the sacrificial metal serves as the anode, and the pipe or tank experiences cathodic protection.
FIGURE 20.24). In moist soil, where corrosion can occur, the sacrificial metal serves as the anode, and the pipe or tank experiences cathodic protection.
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
Based on the values in Table 20.1, which of these metals could provide cathodic protection to iron: Al, Cu, Ni, Zn?