CHEMISTRY THE CENTRAL SCIENCE
20 ELECTRO-CHEMISTRY
EXERCISES
VISUALIZING CONCEPTS
20.1 In the Brønsted-Lowry concept of acids and bases, acid–base reactions are viewed as proton-transfer reactions. The stronger the acid, the weaker is its conjugate base. In what ways are redox reactions analogous? [Sections 20.1 and 20.2]
20.2 You may have heard that “antioxidants” are good for your health. Based on what you have learned in this chapter, what do you deduce an “antioxidant” is? [Sections 20.1 and 20.2]
20.3 The diagram that follows represents a molecular view of a process occurring at an electrode in a voltaic cell.
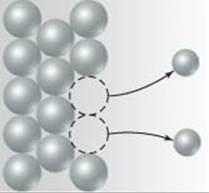
(a) Does the process represent oxidation or reduction? (b) Is the electrode the anode or cathode? (c) Why are the atoms in the electrode represented by larger spheres than the ions in the solution? [Section 20.3]
20.4 Assume that you want to construct a voltaic cell that uses the following half-reactions:
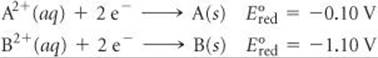
You begin with the incomplete cell pictured here in which the electrodes are immersed in water.
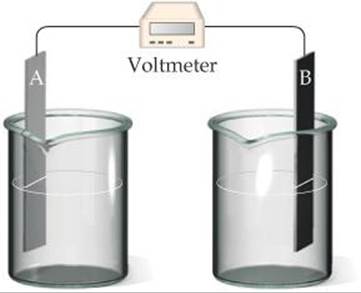
(a) What additions must you make to the cell for it to generate a standard emf? (b) Which electrode functions as the cathode? (c) Which direction do electrons move through the external circuit? (d) What voltage will the cell generate under standard conditions? [Sections 20.3 and20.4]
20.5 For a spontaneous reaction A(aq) + B(aq) → A–(aq) + B+(aq), answer the following questions:
(a) If you made a voltaic cell out of this reaction, what half-reaction would be occurring at the cathode, and what half-reaction would be occurring at the anode?
(b) Which half-reaction from (a) is higher in potential energy?
(c) What is the sign of ![]() ? [Section 20.3]
? [Section 20.3]
20.6 Consider the following table of standard electrode potentials for a series of hypothetical reactions in aqueous solution:
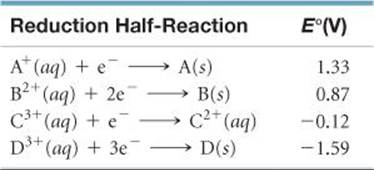
(a) Which substance is the strongest oxidizing agent? Which is weakest?
(b) Which substance is the strongest reducing agent? Which is weakest?
(c) Which substance(s) can oxidize C2+? [Sections 20.4 and 20.5]
20.7 Consider a redox reaction for which E° is a negative number.
(a) What is the sign of ΔG° for the reaction?
(b) Will the equilibrium constant for the reaction be larger or smaller than 1?
(c) Can an electrochemical cell based on this reaction accomplish work on its surroundings? [Section 20.5]
20.8 Consider the following voltaic cell:
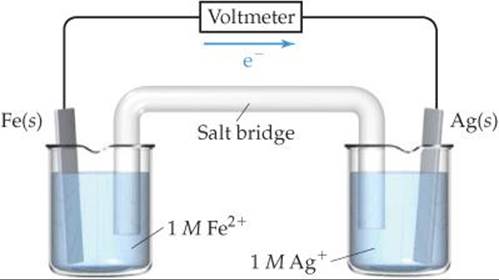
(a) Which electrode is the cathode?
(b) How would you determine the standard emf generated by this cell?
(c) What is the change in the cell voltage when the ion concentrations in the cathode half-cell are increased by a factor of 10?
(d) What is the change in the cell voltage when the ion concentrations in the anode half-cell are increased by a factor of 10? [Sections 20.4 and 20.6]
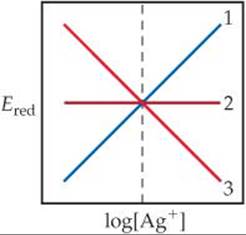
20.9 Consider the half-reaction Ag+(aq) + e– → Ag(s). (a) Which of the lines in the following diagram indicates how the reduction potential varies with the concentration of Ag+? (b) What is the value of Ered when log[Ag+] = 0? [Section 20.6]
20.10 Draw a generic picture of a fuel cell. What is the main difference between it and a battery, regardless of the redox reactions that occur inside? [Section 20.7]
20.11 How does a zinc coating on iron protect the iron from unwanted oxidation? [Section 20.8]
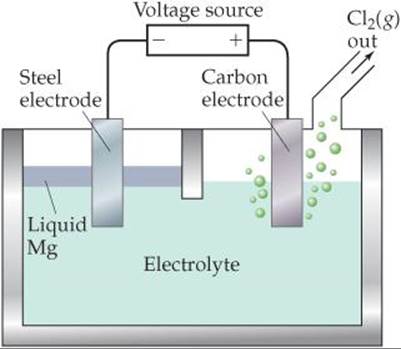
20.12 Magnesium is produced commercially by electrolysis from a molten salt using a cell similar to the one shown here. (a) What salt is used as the electrolyte? (b) Which electrode is the anode, and which one is the cathode? (c) Write the overall cell reaction and individual half-reactions. (d) What precautions would need to be taken with respect to the magnesium formed? [Section 20.9]
OXIDATION-REDUCTION REACTIONS (section 20.1)
20.13 (a) What is meant by the term oxidation? (b) On which side of an oxidation half-reaction do the electrons appear? (c) What is meant by the term oxidant? (d) What is meant by the term oxidizing agent?
20.14 (a) What is meant by the term reduction? (b) On which side of a reduction half-reaction do the electrons appear? (c) What is meant by the term reductant? (d) What is meant by the term reducing agent?
______
20.15 Indicate whether each of the following statements is true or false:
(a) If something is oxidized, it is formally losing electrons.
(b) For the reaction Fe3+(aq) + Co2+(aq) → Fe2+(aq) + Co3+(aq), Fe3+(aq) is the reducing agent and Co2+(aq) is the oxidizing agent.
(c) If there are no changes in the oxidation state of the reac-tants or products of a particular reaction, that reaction is not a redox reaction.
20.16 Indicate whether each of the following statements is true or false:
(a) If something is reduced, it is formally losing electrons.
(b) A reducing agent gets oxidized as it reacts.
(c) Oxidizing agents can convert CO into CO2.
______
20.17 In each of the following balanced oxidation-reduction equations, identify those elements that undergo changes in oxidation number and indicate the magnitude of the change in each case.
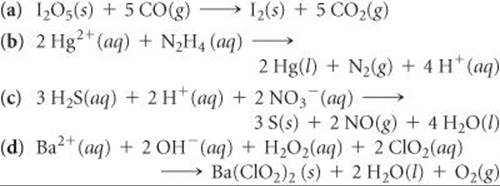
20.18 Indicate whether the following balanced equations involve oxidation-reduction. If they do, identify the elements that undergo changes in oxidation number.
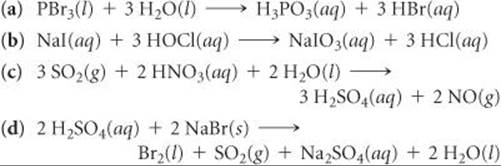
BALANCING OXIDATION-REDUCTION REACTIONS (section 20.2)
20.19 At 900 °C titanium tetrachloride vapor reacts with molten magnesium metal to form solid titanium metal and molten magnesium chloride. (a) Write a balanced equation for this reaction. (b) What is being oxidized, and what is being reduced? (c) Which substance is the reductant, and which is the oxidant?
20.20 Hydrazine (N2H4) and dinitrogen tetroxide (N2O4) form a self-igniting mixture that has been used as a rocket propellant. The reaction products are N2 and H2O. (a) Write a balanced chemical equation for this reaction. (b) What is being oxidized, and what is being reduced? (c)Which substance serves as the reducing agent and which as the oxidizing agent?
______
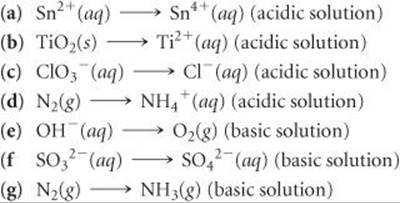
20.21 Complete and balance the following half-reactions. In each case indicate whether the half-reaction is an oxidation or a reduction.
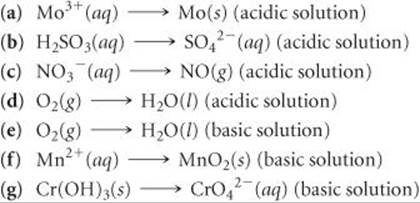
20.22 Complete and balance the following half-reactions. In each case indicate whether the half-reaction is an oxidation or a reduction.
______
20.23 Complete and balance the following equations, and identify the oxidizing and reducing agents:
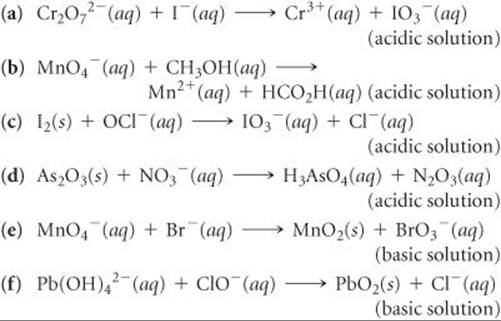
20.24 Complete and balance the following equations, and identify the oxidizing and reducing agents. (Recall that the O atoms in hydrogen peroxide, H2O2, have an atypical oxidation state.)
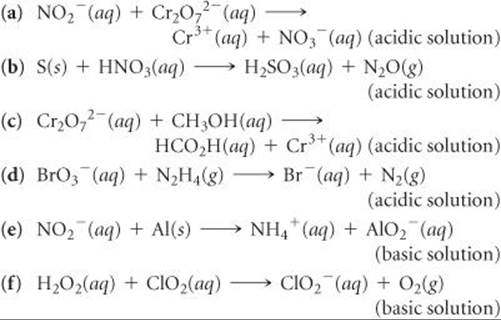
VOLTAIC CELLS (section 20.3)
20.25 (a) What are the similarities and differences between Figure 20.3 and Figure 20.4? (b) Why are Na+ ions drawn into the cathode half-cell as the voltaic cell shown in Figure 20.5 operates?
20.26 (a) What is the role of the porous glass disc shown in Figure 20.4? (b) Why do NO3– ions migrate into the anode half-cell as the voltaic cell shown in Figure 20.5 operates?
______
20.27 A voltaic cell similar to that shown in Figure 20.5 is constructed. One electrode half-cell consists of a silver strip placed in a solution of AgNO3, and the other has an iron strip placed in a solution of FeCl2. The overall cell reaction is
![]()
(a) What is being oxidized, and what is being reduced?
(b) Write the half-reactions that occur in the two half-cells.
(c) Which electrode is the anode, and which is the cathode?
(d) Indicate the signs of the electrodes. (e) Do electrons flow from the silver electrode to the iron electrode or from the iron to the silver? (f) In which directions do the cations and anions migrate through the solution?
20.28 A voltaic cell similar to that shown in Figure 20.5 is constructed. One half-cell consists of an aluminum strip placed in a solution of Al(NO3)3, and the other has a nickel strip placed in a solution of NiSO4. The overall cell reaction is
![]()
(a) What is being oxidized, and what is being reduced?
(b) Write the half-reactions that occur in the two half-cells.
(c) Which electrode is the anode, and which is the cathode? (d) Indicate the signs of the electrodes. (e) Do electrons flow from the aluminum electrode to the nickel electrode or from the nickel to the aluminum? (f) In which directions do the cations and anions migrate through the solution? Assume the Al is not coated with its oxide.
CELL POTENTIALS UNDER STANDARD CONDITIONS (section 20.4)
20.29 (a) What does the term electromotive force mean? (b) What is the definition of the volt? (c) What does the term cell potential mean?
20.30 (a) Which electrode of a voltaic cell, the cathode or the anode, corresponds to the higher potential energy for the electrons? (b) What are the units for electrical potential? How does this unit relate to energy expressed in joules? (c) What is special about a standard cell potential?
______
20.31 (a) Write the half-reaction that occurs at a hydrogen electrode in acidic aqueous solution when it serves as the cathode of a voltaic cell. (b) What is standard about the standard hydrogen electrode? (c) What is the role of the platinum foil in a standard hydrogen electrode?
20.32 (a) Write the half-reaction that occurs at a hydrogen electrode in acidic aqueous solution when it serves as the anode of a voltaic cell. (b) The platinum electrode in a standard hydrogen electrode is specially prepared to have a large surface area. Why is this important? (c) Sketch a standard hydrogen electrode.
______
20.33 (a) What is a standard reduction potential? (b) What is the standard reduction potential of a standard hydrogen electrode?
20.34 (a) Why is it impossible to measure the standard reduction potential of a single half-reaction? (b) Describe how the standard reduction potential of a half-reaction can be determined.
______
20.35 A voltaic cell that uses the reaction
![]()
has a measured standard cell potential of + 1.19 V. (a) Wr i te the two half-cell reactions. (b) By using data from Appendix E, determine ![]() for the reduction of Tl3+(aq) to Tl+(aq). (c) Sketch the voltaic cell, label the anode and cathode, and indicate the direction of electron flow.
for the reduction of Tl3+(aq) to Tl+(aq). (c) Sketch the voltaic cell, label the anode and cathode, and indicate the direction of electron flow.
20.36 A voltaic cell that uses the reaction
![]()
has a measured standard cell potential of +1.03 V. (a) Wr i te the two half-cell reactions. (b) By using data from Appendix E, determine ![]() for the reaction involving Pd. (c) Sketch the voltaic cell, label the anode and cathode, and indicate the direction of electron flow.
for the reaction involving Pd. (c) Sketch the voltaic cell, label the anode and cathode, and indicate the direction of electron flow.
______
20.37 Using standard reduction potentials (Appendix E), calculate the standard emf for each of the following reactions:
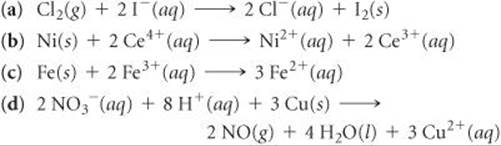
20.38 Using data in Appendix E, calculate the standard emf for each of the following reactions:
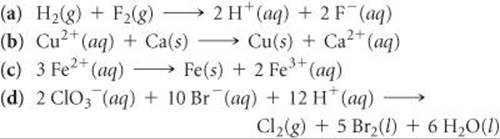
______
20.39 The standard reduction potentials of the following half-reactions are given in Appendix E:
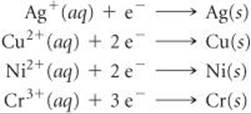
(a) Determine which combination of these half-cell reactions leads to the cell reaction with the largest positive cell potential and calculate the value. (b) Determine which combination of these half-cell reactions leads to the cell reaction with the smallest positive cell potential and calculate the value.
20.40 Given the following half-reactions and associated standard reduction potentials:
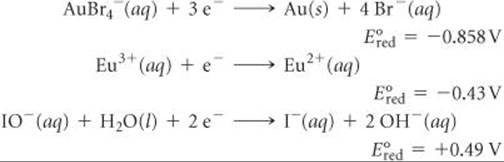
(a) Write the equation for the combination of these half-cell reactions that leads to the largest positive emf and calculate the value. (b) Write the equation for the combination of half-cell reactions that leads to the smallest positive emf and calculate that value.
______
20.41 A 1 M solution of Cu(NO3)2 is placed in a beaker with a strip of Cu metal. A 1 M solution of SnSO4 is placed in a second beaker with a strip of Sn metal. A salt bridge connects the two beakers, and wires to a voltmeter link the two metal electrodes. (a) Which electrode serves as the anode and which as the cathode? (b) Which electrode gains mass and which loses mass as the cell reaction proceeds? (c) Write the equation for the overall cell reaction. (d) What is the emf generated by the cell under standard conditions?
20.42 A voltaic cell consists of a strip of cadmium metal in a solution of Cd(NO3)2 in one beaker, and in the other beaker a platinum electrode is immersed in a NaCl solution, with Cl2 gas bubbled around the electrode. A salt bridge connects the two beakers. (a) Which electrode serves as the anode and which as the cathode? (b) Does the Cd electrode gain or lose mass as the cell reaction proceeds? (c) Write the equation for the overall cell reaction. (d) What is the emf generated by the cell under standard conditions?
STRENGTHS OF OXIDIZING AND REDUCING AGENTS (section 20.4)
20.43 From each of the following pairs of substances, use data in Ap pendix E to choose the one that is the stronger reducing agent:
(a) Fe(s) or Mg(s)
(b) Ca(s) or Al(s)
(c) H2(g, acidic solution) or H2S(g)
(d) BrO3–(aq) or IO3–(aq)
20.44 From each of the following pairs of substances, use data in Ap pendix E to choose the one that is the stronger oxidizing agent:
(a) Cl2(g) or Br2(l)
(b) Zn2+(aq) or Cd2+(aq)
(c) Cl–(aq) or ClO3–(aq)
(d) H2O2(aq) or O3(g)
______
20.45 By using the data in Appendix E, determine whether each of the following substances is likely to serve as an oxidant or a reductant: (a) Cl2(g), (b) MnO4–(aq, acidic solution),(c) Ba(s), (d) Zn(s).
20.46 Is each of the following substances likely to serve as an oxidant or a reductant: (a) Ce3+(aq), (b) Ca(s), (c) ClO3–(aq), (d) N2O5(g)?
______
20.47 (a) Assuming standard conditions, arrange the following in order of increasing strength as oxidizing agents in acidic solution: Cr2O72–, H2O2, Cu2+, Cl2, O2. (b) Arrange the following in order of increasing strength as reducing agents in acidic solution: Zn, I–, Sn2+, H2O2, Al.
20.48 Based on the data in Appendix E, (a) which of the following is the strongest oxidizing agent and which is the weakest in acidic solution:, Br2, H2O2, Zn, Cr2O72–? (b) Which of the following is the strongest reducing agent, and which is the weakest in acidic solution: F–, Zn, N2H5+, I2, NO?
______
20.49 The standard reduction potential for the reduction of Eu3+(aq) to Eu2+(aq) is –0.43 V. Using Appendix E, which of the following substances is capable of reducing Eu3+(aq) to Eu2+(aq) under standard conditions: Al, Co, H2O2, N2H5+, H2C2O4?
20.50 The standard reduction potential for the reduction of RuO4-(aq) to RuO42–(aq) is +0.59 V. By using Appendix E, which of the following substances can oxidize RuO42–(aq) to RuO4–(aq) under standard conditions: Br2(l), BrO3–(aq), Mn2+(aq), O2(g), Sn2+(aq)?
FREE ENERGY AND REDOX REACTIONS (section 20.5)
20.51 Given the following reduction half-reactions:
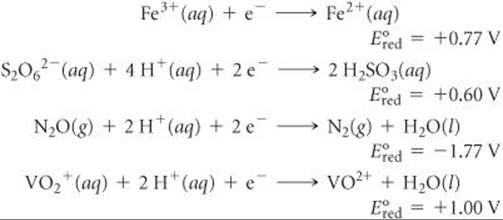
(a) Write balanced chemical equations for the oxidation of Fe2+(aq) by S2O62–(aq), by N2O(aq), and by VO2+(aq). (b) Calculate ΔG° for each reaction at 298 K. (c) Calculate the equilibrium constant K for each reaction at 298 K.
20.52 For each of the following reactions, write a balanced equation, calculate the standard emf, calculate ΔG° at 298 K, and calculate the equilibrium constant K at 298 K. (a) Aqueous iodide ion is oxidized to I2(s) by Hg22+(aq). (b) In acidic solution, copper(I) ion is oxidized to copper(II) ion by nitrate ion. (c) In basic solution, Cr(OH)3(s) is oxidized to CrO42–(aq) by ClO–(aq).
______
20.53 If the equilibrium constant for a two-electron redox reaction at 298 K is 1.5 × 10–4, calculate the corresponding ΔG° and ![]() .
.
20.54 If the equilibrium constant for a one-electron redox reaction at 298 K is 8.7 × 10–4, calculate the corresponding ΔG° and ![]() .
.
______
20.55 Using the standard reduction potentials listed in Appendix E, calculate the equilibrium constant for each of the following reactions at 298 K:
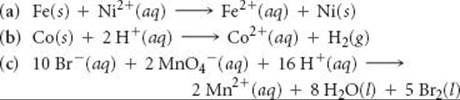
20.56 Using the standard reduction potentials listed in Appendix E, calculate the equilibrium constant for each of the following reactions at 298 K:
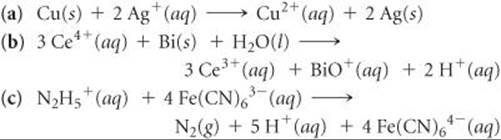
______
20.57 A cell has a standard cell potential of +0.177 V at 298 K. What is the value of the equilibrium constant for the reaction (a) if n = 1? (b) if n = 2? (c) if n = 3?
20.58 At 298 K a cell reaction has a standard cell potential of +0.17 V. The equilibrium constant for the reaction is 5.5 × 105. What is the value of n for the reaction?
______
20.59 A voltaic cell is based on the reaction
![]()
Under standard conditions, what is the maximum electrical work, in joules, that the cell can accomplish if 75.0 g of Sn is consumed?
20.60 Consider the voltaic cell illustrated in Figure 20.5, which is based on the cell reaction
![]()
Under standard conditions, what is the maximum electrical work, in joules, that the cell can accomplish if 50.0 g of copper is formed?
CELL EMF UNDER NONSTANDARD CONDITIONS (section 20.6)
20.61 (a) Under what circumstances is the Nernst equation applicable? (b) What is the numerical value of the reaction quotient, Q, under standard conditions? (c) What happens to the emf of a cell if the concentrations of the reactants are increased?
20.62 (a) A voltaic cell is constructed with all reactants and products in their standard states. Will this condition hold as the cell operates? Explain. (b) Can the Nernst equation be used at temperatures other than room temperature? Explain. (c) What happens to the emf of a cell if the concentrations of the products are increased?
______
20.63 What is the effect on the emf of the cell shown in Figure 20.9, which has the overall reaction Zn(s) + 2 H+(aq) → Zn2+(aq) + H2(g), for each of the following changes? (a) The pressure of the H2 gas is increased in the cathode half-cell. (b) Zinc nitrate is added to the anode half-cell. (c) Sodium hydroxide is added to the cathode half-cell, decreasing [H+]. (d) The surface area of the anode is doubled.
20.64 A voltaic cell utilizes the following reaction:
![]()
What is the effect on the cell emf of each of the following changes? (a) Water is added to the anode half-cell, diluting the solution. (b) The size of the aluminum electrode is increased. (c) A solution of AgNO3 is added to the cathode half-cell, increasing the quantity of Ag+ but not changing its concentration. (d) HCl is added to the AgNO3 solution, precipitating some of the Ag+ as AgCl.
______
20.65 A voltaic cell is constructed that uses the following reaction and operates at 298 K:
![]()
(a) What is the emf of this cell under standard conditions? (b) What is the emf of this cell when [Ni2+] = 3.00 M and [Zn2+] = 0.100 M? (c) What is the emf of the cell when [Ni2+] = 0.200 M and [Zn2+] = 0.900 M?
20.66 A voltaic cell utilizes the following reaction and operates at 298 K:
![]()
(a) What is the emf of this cell under standard conditions? (b) What is the emf of this cell when [Ce4+] = 3.0 M [Ce3+] = 0.10 M, and [Cr3+] = 0.010 M? (c) What is the emf of the cell when [Ce4+] = 0.010 M, [Ce3+] = 2.0 M and [Ce3+] = 1.5 M?
______
20.67 A voltaic cell utilizes the following reaction:
![]()
(a) What is the emf of this cell under standard conditions? (b) What is the emf of this cell when [Fe2+] = 1.3 M, [Fe3+] = 0.010 M, PO2 = 0.50 atm, and the pH of the solution in the cathode half-cell is 3.50?
20.68 A voltaic cell utilizes the following reaction:
![]()
(a) What is the emf of this cell under standard conditions? (b) What is the emf for this cell when [Fe3+] = 3.50 M, PH2 = 0.95 atm, [Fe2+] = 0.0010 M, and the pH in both half-cells is 4.00?
______
20.69 A voltaic cell is constructed with two Zn2+ –Zn electrodes. The two half-cells have [Zn2+] = 1.8 M and [Zn2+] = 1.00 × 10–2M, respectively. (a) Which electrode is the anode of the cell? (b) What is the standard emf of the cell? (c) What is the cell emf for the concentrations given?(d) For each electrode, predict whether [Zn2+] will increase, decrease, or stay the same as the cell operates.
20.70 A voltaic cell is constructed with two silver–silver chloride electrodes, each of which is based on the following half-reaction:
![]()
The two half-cells have [Cl–] = 0.0150 M and [Cl–] = 2.55 M, respectively. (a) Which electrode is the cathode of the cell? (b) What is the standard emf of the cell? (c) What is the cell emf for the concentrations given? (d) For each electrode, predict whether [Cl–] will increase, decrease, or stay the same as the cell operates.
______
20.71 The cell in Figure 20.9 could be used to provide a measure of the pH in the cathode half-cell. Calculate the pH of the cathode half-cell solution if the cell emf at 298 K is measured to be +0.684 V when [Zn2+] = 0.30 M and PH2 = 0.90 atm.
20.72 A voltaic cell is constructed that is based on the following reaction:
![]()
(a) If the concentration of Sn2+ in the cathode half-cell is 1.00 M and the cell generates an emf of +0.22 V, what is the concentration of Pb2+ in the anode half-cell? (b) If the anode half-cell contains [SO42–] = 1.00 M in equilibrium with PbSO4(s), what is the Ksp of PbSO4?
BATTERIES AND FUEL CELLS (section 20.7)
20.73 (a) What happens to the emf of a battery as it is used? Why does this happen? (b) The AA-size and D-size alkaline batteries are both 1.5-V batteries that are based on the same electrode reactions. What is the major difference between the two batteries? What performance feature is most affected by this difference?
20.74 (a) Suggest an explanation for why liquid water is needed in an alkaline battery. (b) What is the advantage of using highly concentrated or solid reactants in a voltaic cell?
______
20.75 During a period of discharge of a lead-acid battery, 402 g of Pb from the anode is converted into PbSO4(s). (a) What mass of PbO2(s) is reduced at the cathode during this same period? (b) How many coulombs of electrical charge are transferred from Pb to PbO2?
20.76 During the discharge of an alkaline battery, 4.50 g of Zn is consumed at the anode of the battery. (a) What mass of MnO2 is reduced at the cathode during this discharge? (b) How many coulombs of electrical charge are transferred from Zn to MnO2?
______
20.77 Heart pacemakers are often powered by lithium–silver chro-mate “button” batteries. The overall cell reaction is
![]()
(a) Lithium metal is the reactant at one of the electrodes of the battery. Is it the anode or the cathode? (b) Choose the two half-reactions from Appendix E that most closely approximate the reactions that occur in the battery. What standard emf would be generated by a voltaic cell based on these half-reactions? (c) The battery generates an emf of +3.5 V. How close is this value to the one calculated in part (b)? (d) Calculate the emf that would be generated at body temperature, 37 °C. How does this compare to the emf you calculated in part (b)?
20.78 Mercuric oxide dry-cell batteries are often used where a high-energy density is required, such as in watches and cameras. The two half-cell reactions that occur in the battery are

(a) Write the overall cell reaction. (b) The value of ![]() for the cathode reaction is +0.098 V. The overall cell potential is +1.35 V. Assuming that both half-cells operate under standard conditions, what is the standard reduction potential for the anode reaction? (c) Why is the potential of the anode reaction different than would be expected if the reaction occurred in an acidic medium?
for the cathode reaction is +0.098 V. The overall cell potential is +1.35 V. Assuming that both half-cells operate under standard conditions, what is the standard reduction potential for the anode reaction? (c) Why is the potential of the anode reaction different than would be expected if the reaction occurred in an acidic medium?
______
20.79 (a) Suppose that an alkaline battery was manufactured using cadmium metal rather than zinc. What effect would this have on the cell emf? (b) What environmental advantage is provided by the use of nickel-metal-hydride batteries over nickel-cadmium batteries?
20.80 (a) The nonrechargeable lithium batteries used for photography use lithium metal as the anode. What advantages might be realized by using lithium rather than zinc, cadmium, lead, or nickel? (b) The rechargeable lithium-ion battery does not use lithium metal as an electrode material. Nevertheless, it still has a substantial advantage over nickel-based batteries. Suggest an explanation.
______
20.81 The hydrogen-oxygen fuel cell has a standard emf of 1.23 V. What advantages and disadvantages there to using this device as a source of power compared to a 1.55-V alkaline battery?
20.82 (a) What is the difference between a battery and a fuel cell? (b) Can the “fuel” of a fuel cell be a solid? Explain.
CORROSION (section 20.8)
20.83 (a) Write the anode and cathode reactions that cause the cor rosion of iron metal to aqueous iron(II). (b) Write the balanced half-reactions involved in the air oxidation of Fe2+(aq) to Fe2O3 · 3 H2O.
20.84 (a) Based on standard reduction potentials, would you expect copper metal to oxidize under standard conditions in the presence of oxygen and hydrogen ions? (b) When the Statue of Liberty was refurbished, Teflon spacers were placed between the iron skeleton and the copper metal on the surface of the statue. What role do these spacers play?
______
20.85 (a) Magnesium metal is used as a sacrificial anode to protect underground pipes from corrosion. Why is the magnesium referred to as a “sacrificial anode”? (b) Looking in Appendix E, suggest what metal the underground pipes could be made from in order for magnesium to be successful as a sacrificial anode.
20.86 An iron object is plated with a coating of cobalt to protect against corrosion. Does the cobalt protect iron by cathodic protection? Explain.
______
20.87 A plumber's handbook states that you should not connect a brass pipe directly to a galvanized steel pipe because electrochemical reactions between the two metals will cause corrosion. The handbook recommends you use instead an insulating fitting to connect them. Brass is a mixture of copper and zinc. What spontaneous redox reaction(s) might cause the corrosion? Justify your answer with standard emf calculations.
20.88 A plumber's handbook states that you should not connect a copper pipe directly to a steel pipe because electrochemical reactions between the two metals will cause corrosion. The handbook recommends you use instead an insulating fitting to connect them. What spontaneous redox reaction(s) might cause the corrosion? Justify your answer with standard emf calculations.
ELECTROLYSIS (section 20.9)
20.89 (a) What is electrolysis? (b) Are electrolysis reactions thermo-dynamically spontaneous? Explain. (c) What process occurs at the anode in the electrolysis of molten NaCl? (d) Why is sodium metal not obtained when an aqueous solution of NaCl undergoes electrolysis?
20.90 (a) What is an electrolytic cell? (b) The negative terminal of a voltage source is connected to an electrode of an electrolytic cell. Is the electrode the anode or the cathode of the cell? Explain. (c) The electrolysis of water is often done with a small amount of sulfuric acid added to the water. What is the role of the sulfuric acid? (d) Why are active metals such as Al obtained by electrolysis using molten salts rather than aqueous solutions?
______
20.91 (a) A Cr3+(aq) solution is electrolyzed, using a current of 7.60 A. What mass of Cr(s) is plated out after 2.00 days? (b) What amperage is required to plate out 0.250 mol Cr from a Cr3+ solution in a period of 8.00 h?
20.92 Metallic magnesium can be made by the electrolysis of molten MgCl2. (a) What mass of Mg is formed by passing a current of 4.55 A through molten MgCl2, for 4.50 days? (b) How many minutes are needed to plate out 25.00 g Mg from molten MgCl2 using 3.50 A of current?
______
20.93 (a) Calculate the mass of Li formed by electrolysis of molten LiCl by a current of 7.5 × 10–4 A flowing for a period of 24 h. Assume the electrolytic cell is 85% efficient. (b) What is the minimum voltage required to drive the reaction?
20.94 Elemental calcium is produced by the electrolysis of molten CaCl2. (a) What mass of calcium can be produced by this process if a current of 7.5 × 10–3 A is applied for 48 h? Assume that the electrolytic cell is 68% efficient. (b) What is the minimum voltage needed to cause the electrolysis?
______
20.95 Metallic gold is collected from below the anode when crude copper metal is refined by electrolysis. Explain this behavior.
20.96 The crude copper that is subjected to electrorefining contains tellurium as an impurity. The standard reduction potential between tellurium and its lowest common oxidation state, Te4+, is
![]()
Given this information, describe the probable fate of tellurium impurities during electrorefining. Do the impurities fall to the bottom of the refining bath, unchanged, as copper is oxidized, or do they go into solution? If they go into solution, do they plate out on the cathode?
ADDITIONAL EXERCISES
20.97 A disproportionation reaction is an oxidation-reduction reaction in which the same substance is oxidized and reduced. Complete and balance the following disproportionation reactions:
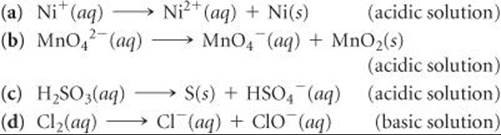
20.98 This oxidation-reduction reaction in acidic solution is spontaneous:
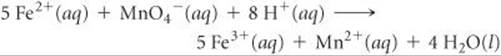
A solution containing KMnO4 and H2SO4 is poured into one beaker, and a solution of FeSO4 is poured into another. A salt bridge is used to join the beakers. A platinum foil is placed in each solution, and a wire that passes through a voltmeter connects the two solutions. (a) Sketch the cell, indicating the anode and the cathode, the direction of electron movement through the external circuit, and the direction of ion migrations through the solutions. (b) Sketch the process that occurs at the atomic level at the surface of the anode. (c) Calculate the emf of the cell under standard conditions. (d) Calculate the emf of the cell at 298 K when the concentrations are the following: pH = 0.0, [Fe2+] = 0.10 M, [MnO4–] = 1.50 M, [Fe3+] = 2.5 × 10–4M, [Mn2+] = 0.001 M.
20.99 A common shorthand way to represent a voltaic cell is
anode|anode solution || cathode solution|cathode
A double vertical line represents a salt bridge or a porous barrier. A single vertical line represents a change in phase, such as from solid to solution. (a) Write the half-reactions and overall cell reaction represented by ![]() sketch the cell. (b) Write the half-reactions and overall cell reaction represented by
sketch the cell. (b) Write the half-reactions and overall cell reaction represented by ![]() ; sketch the cell. (c) Using the notation just described, represent a cell based on the following reaction:
; sketch the cell. (c) Using the notation just described, represent a cell based on the following reaction:
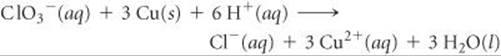
Pt is used as an inert electrode in contact with the ClO3– and Cl–. Sketch the cell.
20.100 Predict whether the following reactions will be spontaneous in acidic solution under standard conditions: (a) oxidation of Sn to Sn2+ by I2 (to form I–), (b) reduction of Ni2+ to Ni by I–(to form I2), (c) reduction of Ce4+ to Ce3+ by H2O2, (d) reduction of Cu2+ to Cu by Sn2+ (to form Sn4+).
[20.101] Gold exists in two common positive oxidation states, +1 and +3. The standard reduction potentials for these oxidation states are
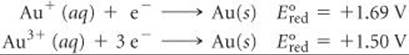
(a) Can you use these data to explain why gold does not tarnish in the air? (b) Suggest several substances that should be strong enough oxidizing agents to oxidize gold metal. (c) Miners obtain gold by soaking gold-containing ores in an aqueous solution of sodium cyanide. A very soluble complex ion of gold forms in the aqueous solution because of the redox reaction
![]()
What is being oxidized and what is being reduced in this reaction? (d) Gold miners then react the basic aqueous product solution from part (c) with Zn dust to get gold metal. Write a balanced redox reaction for this process. What is being oxidized, and what is being reduced?
20.102 A voltaic cell is constructed from an Ni2+(aq)-Ni(s) half-cell and an Ag+(aq)-Ag(s) half-cell. The initial concentration of Ni2+(aq) in the Ni2+-Ni half-cell is [Ni2+] = 0.0100 M. The initial cell voltage is +1.12 V. (a) By using data in Table 20.1, calculate the standard emf of this voltaic cell. (b) Will the concentration of Ni2+(aq) increase or decrease as the cell operates? (c) What is the initial concentration of Ag +(aq) in the Ag+-Ag half-cell?
[20.103] A voltaic cell is constructed that uses the following half-cell reactions:
![]()
The cell is operated at 298 K with [Cu+] = 0.25 M and [I–] = 3.5 M. (a) Determine E for the cell at these concentrations. (b) Which electrode is the anode of the cell? (c) Is the answer to part (b) the same as it would be if the cell were operated under standard conditions? (d) If [Cu+] was equal to 0.15 M, at what concentration of I would the cell have zero potential?
20.104 Using data from Appendix E, calculate the equilibrium constant for the disproportionation of the copper(I) ion at room temperature: ![]() .
.
20.105 (a) Write the reactions for the discharge and charge of a nickel-cadmium (nicad) rechargeable battery. (b) Given the following reduction potentials, calculate the standard emf of the cell:
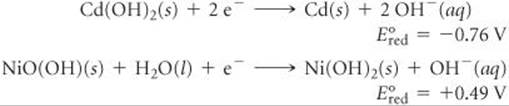
(c) A typical nicad voltaic cell generates an emf of +1.30 V. Why is there a difference between this value and the one you calculated in part (b)? (d) Calculate the equilibrium constant for the overall nicad reaction based on this typical emf value.
20.106 The capacity of batteries such as the typical AA alkaline battery is expressed in units of milliamp-hours (mAh). An AA alkaline battery yields a nominal capacity of 2850 mAh. (a) What quantity of interest to the consumer is being expressed by the units of mAh? (b) The starting voltage of a fresh alkaline battery is 1.55 V. The voltage decreases during discharge and is 0.80 V when the battery has delivered its rated capacity. If we assume that the voltage declines linearly as current is withdrawn, estimate the total maximum electrical work the battery could perform during discharge.
20.107 If you were going to apply a small potential to a steel ship resting in the water as a means of inhibiting corrosion, would you apply a negative or a positive charge? Explain.
[20.108] (a) How many coulombs are required to plate a layer of chromium metal 0.25 mm thick on an auto bumper with a total area of 0.32 m2 from a solution containing CrO42–? The density of chromium metal is 7.20 g/cm3. (b) What current flow is required for this electroplating if the bumper is to be plated in 10.0 s? (c) If the external source has an emf of +6.0 V and the electrolytic cell is 65% efficient, how much electrical power is expended to electroplate the bumper?
20.109 Magnesium is obtained by electrolysis of molten MgCl2. (a) Why is an aqueous solution of MgCl2 not used in the electrolysis? (b) Several cells are connected in parallel by very large copper buses that convey current to the cells. Assuming that the cells are 96% efficient in producing the desired products in electrolysis, what mass of Mg is formed by passing a current of 97,000 A for a period of 24 hr?
20.110 Calculate the number of kilowatt-hours of electricity required to produce 1.0 × 103 kg (1 metric ton) of aluminum by electrolysis of Al3+ if the applied voltage is 4.50 V and the process is 45% efficient.
20.111 Some years ago a unique proposal was made to raise the Titanic. The plan involved placing pontoons within the ship using a surface-controlled submarine-type vessel. The pontoons would contain cathodes and would be filled with hydrogen gas formed by the electrolysis of water. It has been estimated that it would require about 7 × 108 mol of H2 to provide the buoyancy to lift the ship (J. Chem. Educ., 1973, Vol. 50, 61). (a) How many coulombs of electrical charge would be required? (b) What is the minimum voltage required to generate H2and O2 if the pressure on the gases at the depth of the wreckage (2 mi) is 300 atm? (c) What is the minimum electrical energy required to raise the Titanic by electrolysis? (d) What is the minimum cost of the electrical energy required to generate the necessary H2 if the electricity costs 85 cents per kilowatt-hour to generate at the site?
INTEGRATIVE EXERCISES
20.112 The Haber process is the principal industrial route for converting nitrogen into ammonia:
![]()
(a) What is being oxidized, and what is being reduced? (b) Using the thermodynamic data in Appendix C, calculate the equilib rium constant for the process at room temperature. (c) Calculate the standard emf of the Haber process at room temperature.
[20.113] In a galvanic cell the cathode is an Ag+ (1.00 M)/Ag(s) half-cell. The anode is a standard hydrogen electrode immersed in a buffer solution containing 0.10 Mbenzoic acid (C6H5COOH) and 0.050 M sodium benzoate (C6H5COO– Na+). The measured cell voltage is 1.030 V. What is the pKa of benzoic acid?
20.114 Consider the general oxidation of a species A in solution: ![]() . The term oxidation potential is sometimes used to describe the ease with which species A is oxidized—the easier a species is to oxidize, the greater its oxidation potential. (a) What is the relationship between the standard oxidation potential of A and the standard reduction potential of A+? (b) Which of the metals listed in Table 4.5 has the highest standard oxidation potential? Which has the lowest? (c) For a series of substances, the trend in oxidation potential is often related to the trend in the first ionization energy. Explain why this relationship makes sense.
. The term oxidation potential is sometimes used to describe the ease with which species A is oxidized—the easier a species is to oxidize, the greater its oxidation potential. (a) What is the relationship between the standard oxidation potential of A and the standard reduction potential of A+? (b) Which of the metals listed in Table 4.5 has the highest standard oxidation potential? Which has the lowest? (c) For a series of substances, the trend in oxidation potential is often related to the trend in the first ionization energy. Explain why this relationship makes sense.
20.115 A voltaic cell is based on Ag+(aq)/Ag(s) and Fe3+(aq)/Fe2+(aq) half-cells. (a) What is the standard emf of the cell? (b) Which reaction occurs at the cathode and which at the anode of the cell? (c) Use S° values in Appendix C and the relationship between cell potential and free-energy change to predict whether the standard cell potential increases or decreases when the temperature is raised above 25°C.
20.116 Hydrogen gas has the potential as a clean fuel in reaction with oxygen. The relevant reaction is
![]()
Consider two possible ways of utilizing this reaction as an electrical energy source: (i) Hydrogen and oxygen gases are combusted and used to drive a generator, much as coal is currently used in the electric power industry; (ii) hydrogen and oxygen gases are used to generate electricity directly by using fuel cells that operate at 85 °C. (a) Use data in Appendix C to calculate ΔH° and ΔS° for the reaction. We will assume that these values do not change appreciably with temperature. (b) Based on the values from part (a), what trend would you expect for the magnitude of ΔG for the reaction as the temperature increases? (c) What is the significance of the change in the magnitude of ΔG with temperature with respect to the utility of hydrogen as a fuel? (d) Based on the analysis here, would it be more efficient to use the combustion method or the fuel-cell method to generate electrical energy from hydrogen?
20.117 Cytochrome, a complicated molecule that we will represent as CyFe2+, reacts with the air we breathe to supply energy required to synthesize adenosine triphosphate (ATP). The body uses ATP as an energy source to drive other reactions. (Section 19.7) At pH 7.0 the following reduction potentials pertain to this oxidation of CyFe2+:
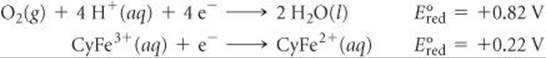
(a) What is ΔG for the oxidation of CyFe2+ by air? (b) If the synthesis of 1.00 mol of ATP from adenosine diphosphate (ADP) requires a ΔG of 37.7 kJ, how many moles of ATP are synthesized per mole of O2?
[20.118] The standard potential for the reduction of AgSCN(s) is +0.0895 V.
![]()
Using this value and the electrode potential for Ag+(aq), calculate the Ksp for AgSCN.
[20.119] The Ksp value for PbS(s) is 8.0 × 10–28. By using this value together with an electrode potential from Appendix E, determine the value of the standard reduction potential for the reaction
![]()
[20.120] A student designs an ammeter (a device that measures electrical current) that is based on the electrolysis of water into hydrogen and oxygen gases. When electrical current of unknown magnitude is run through the device for 2.00 min, 12.3 mL of water-saturated H2(g) is collected. The temperature of the system is 25.5 °C, and the atmospheric pressure is 768 torr. What is the magnitude of the current in amperes?