CHEMISTRY THE CENTRAL SCIENCE
21 NUCLEAR CHEMISTRY
21.2 PATTERNS OF NUCLEAR STABILITY
No single rule allows us to predict whether a particular nucleus is radioactive and, if it is, how it might decay. However, several empirical observations can help us predict the stability of a nucleus.
Neutron-to-Proton Ratio
Because like charges repel each other, it may seem surprising that a large number of protons can reside within the small volume of the nucleus. At close distances, however, a strong force of attraction, called the nuclear force, exists between nucleons. Neutrons are intimately involved in this attractive force. All nuclei other than ![]() contain neutrons. As the number of protons in a nucleus increases, there is an ever greater need for neutrons to counteract the proton-proton repulsions. Stable nuclei with atomic numbers up to about 20 have approximately equal numbers of neutrons and protons. For nuclei with atomic number above 20, the number of neutrons exceeds the number of protons. Indeed, the number of neutrons necessary to create a stable nucleus increases more rapidly than the number of protons. Thus, the neutron-to-proton ratios of stable nuclei increase with increasing atomic number, as illustrated by the most common isotopes of carbon,
contain neutrons. As the number of protons in a nucleus increases, there is an ever greater need for neutrons to counteract the proton-proton repulsions. Stable nuclei with atomic numbers up to about 20 have approximately equal numbers of neutrons and protons. For nuclei with atomic number above 20, the number of neutrons exceeds the number of protons. Indeed, the number of neutrons necessary to create a stable nucleus increases more rapidly than the number of protons. Thus, the neutron-to-proton ratios of stable nuclei increase with increasing atomic number, as illustrated by the most common isotopes of carbon, ![]() (n/p = 1), manganese,
(n/p = 1), manganese, ![]() (n/p = 1.20), and gold,
(n/p = 1.20), and gold, ![]() (n/p = 1.49).
(n/p = 1.49).
The dark blue dots in ![]() FIGURE 21.2 represent stable (nonradioactive) isotopes. The region of the graph covered by these dark blue dots is known as the belt of stability. The belt of stability ends at element 83 (bismuth), which means that all nuclei with 84 or more protons are radioactive. For example, all isotopes of uranium, Z = 92, are radioactive.
FIGURE 21.2 represent stable (nonradioactive) isotopes. The region of the graph covered by these dark blue dots is known as the belt of stability. The belt of stability ends at element 83 (bismuth), which means that all nuclei with 84 or more protons are radioactive. For example, all isotopes of uranium, Z = 92, are radioactive.
The type of radioactive decay that a particular radionuclide undergoes depends largely on how its neutron-to-proton ratio compares with those of nearby nuclei that lie within the belt of stability. We can envision three general situations:
1. Nuclei above the belt of stability (high neutron-to-proton ratios). These neutron-rich nuclei can lower their ratio and thereby move toward the belt of stability by emitting a beta particle because beta emission decreases the number of neutrons and increases the number of protons (Equation 21.3).
2. Nuclei below the belt of stability (low neutron-to-proton ratios). These proton-rich nuclei can increase their ratio and so move closer to the belt of stability by either positron emission or electron capture because both decays increase the number of neutrons and decrease the number of protons (Equations 21.5 and 21.7). Positron emission is more common among lighter nuclei. Electron capture becomes increasingly common as the nuclear charge increases.
3. Nuclei with atomic numbers ≥ 84. These heavy nuclei tend to undergo alpha emission, which decreases both the number of neutrons and the number of protons by two, moving the nucleus diagonally toward the belt of stability.
SAMPLE EXERCISE 21.3 Predicting Modes of Nuclear Decay
Predict the mode of decay of (a) carbon-14, (b) xenon-118.
SOLUTION
Analyze We are asked to predict the modes of decay of two nuclei.
Plan To do this, we must locate the respective nuclei in Figure 21.2 and determine their positions with respect to the belt of stability in order to predict the most likely mode of decay.
Solve
(a) Carbon is element 6. Thus, carbon-14 has 6 protons and 14 – 6 = 8 neutrons, giving it a neutron-to-proton ratio of 1.25. Elements with Z < 20 normally have stable nuclei that contain approximately equal numbers of neutrons and protons (n/p = 1). Thus, carbon-14 is located above the belt of stability and we expect it to decay by emitting a beta particle to lessen the n/p ratio:
![]()
This is indeed the mode of decay observed for carbon-14, a reaction that lowers the n/p ratio from 1.25 to 1.0.
(b) Xenon is element 54. Thus, xenon-118 has 54 protons and 118 – 54 = 64 neutrons, giving it an n/p ratio of 1.18. According to Figure 21.2, stable nuclei in this region of the belt of stability have higher neutron-to-proton ratios than xenon-118. The nucleus can increase this ratio by either positron emission or electron capture:
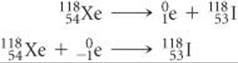
In this case both modes of decay are observed.
Comment Keep in mind that our guidelines do not always work. For example, thorium-233, which we might expect to undergo alpha decay, actually undergoes beta emission. Furthermore, a few radioactive nuclei lie within the belt of stability. Both ![]() and
and ![]() , for example, are stable and lie in the belt of stability.
, for example, are stable and lie in the belt of stability. ![]() , however, which lies between them, is radioactive.
, however, which lies between them, is radioactive.
PRACTICE EXERCISE
Predict the mode of decay of (a) plutonium-239, (b) indium-120.
Answers: (a) α decay, (b) β emission
![]() GO FIGURE
GO FIGURE
Estimate the optimal number of neutrons for a nucleus containing 70 protons.
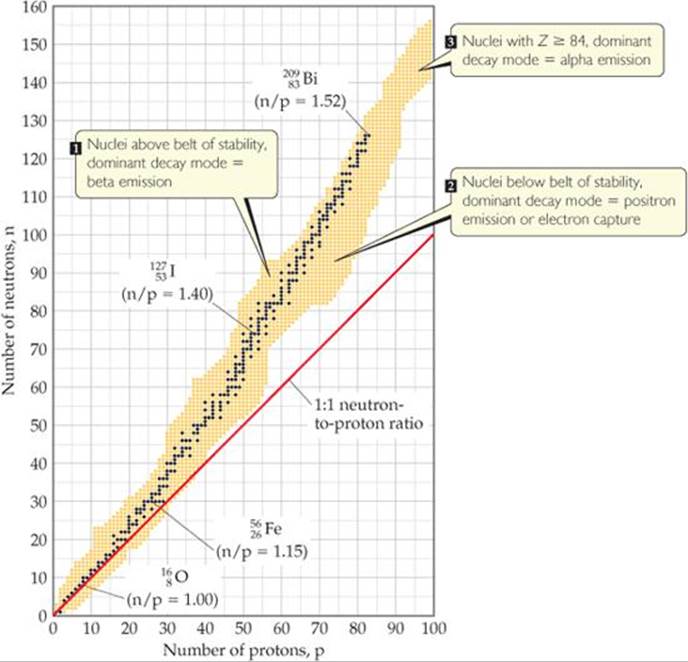
![]() FIGURE 21.2 Stable and radioactive isotopes as a function of numbers of neutrons and protons in a nucleus. The stable nuclei (dark blue dots) define a region known as the belt of stability.
FIGURE 21.2 Stable and radioactive isotopes as a function of numbers of neutrons and protons in a nucleus. The stable nuclei (dark blue dots) define a region known as the belt of stability.
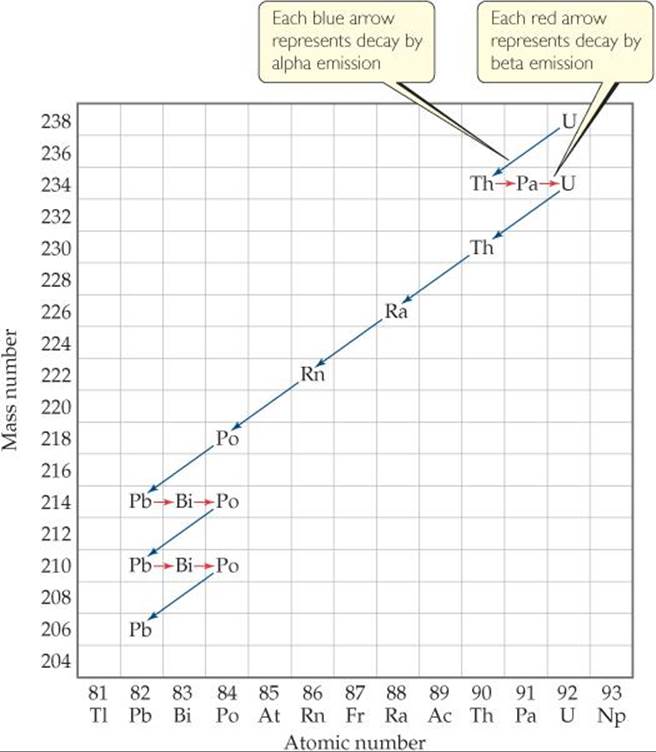
![]() FIGURE 21.3 Nuclear disintegration series for uranium-238. The decay continues until the stable nucleus
FIGURE 21.3 Nuclear disintegration series for uranium-238. The decay continues until the stable nucleus ![]() is formed.
is formed.
Radioactive Series
Some nuclei cannot gain stability by a single emission. Consequently, a series of successive emissions occurs as shown for uranium-238 in ![]() FIGURE 21.3. Decay continues until a stable nucleus—lead-206 in this case—is formed. A series of nuclear reactions that begins with an unstable nucleus and terminates with a stable one is known as a radioactive series or a nuclear disintegration series. Three such series occur in nature: uranium-238 to lead-206, uranium-235 to lead-207, and thorium-232 to lead-208.
FIGURE 21.3. Decay continues until a stable nucleus—lead-206 in this case—is formed. A series of nuclear reactions that begins with an unstable nucleus and terminates with a stable one is known as a radioactive series or a nuclear disintegration series. Three such series occur in nature: uranium-238 to lead-206, uranium-235 to lead-207, and thorium-232 to lead-208.
Further Observations
Two further observations can help us to predict stable nuclei:
• Nuclei with the magic numbers of 2, 8, 20, 28, 50, or 82 protons or 2, 8, 20, 28, 50, 82, or 126 neutrons are generally more stable than nuclei that do not contain these numbers of nucleons.
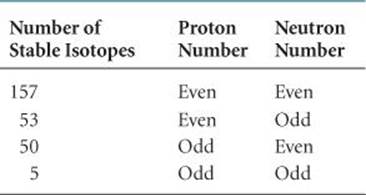
• Nuclei with even numbers of protrons, neutrons, or both are more likely to be stable than those with odd numbers of protons and/or neutrons. Approximately 60% of stable nuclei have an even number of both protons and neutrons, whereas less than 2% have odd numbers of both (![]() TABLE 21.4).
TABLE 21.4).
TABLE 21.4 • Number of Stable Isotopes with Even and Odd Numbers of Protons and Neutrons
These observations can be understood in terms of the shell model of the nucleus, in which nucleons are described as residing in shells analogous to the shell structure for electrons in atoms. Just as certain numbers of electrons correspond to stable filled-shell electron configurations, so also the magic numbers of nucleons represent filled shells in nuclei.
There are several examples of the stability of nuclei with magic numbers of nucleons. For example, the radioactive series in Figure 21.3 ends with the stable ![]() nucleus, which has a magic number of protons (82). Another example is the observation that tin, which has a magic number of protons (50), has ten stable isotopes, more than any other element.
nucleus, which has a magic number of protons (82). Another example is the observation that tin, which has a magic number of protons (50), has ten stable isotopes, more than any other element.
![]() GO FIGURE
GO FIGURE
Among the elements shown here, how many have an even number of protons and fewer than three stable isotopes? How many have an odd number of protons and more than two stable isotopes?
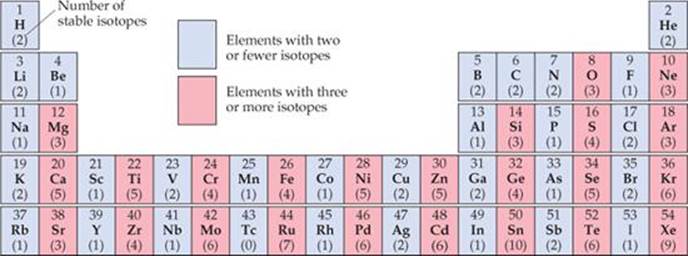
![]() FIGURE 21.4 Number of stable isotopes for elements 1–54.
FIGURE 21.4 Number of stable isotopes for elements 1–54.
Evidence also suggests that pairs of protons and pairs of neutrons have a special stability, analogous to the pairs of electrons in molecules. This evidence accounts for the second observation noted earlier, that stable nuclei with an even number of protons and/or neutrons are far more numerous than those with odd numbers. The preference for even numbers of protons is illustrated in ![]() FIGURE 21.4, which shows the number of stable isotopes for all elements up to Xe. Notice that once we move past nitrogen, the elements with an odd number of protons invariably have fewer stable isotopes than their neighbors with an even number of protons.
FIGURE 21.4, which shows the number of stable isotopes for all elements up to Xe. Notice that once we move past nitrogen, the elements with an odd number of protons invariably have fewer stable isotopes than their neighbors with an even number of protons.
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
What can you say about the number of neutrons in the stable isotopes of fluorine, sodium, aluminum, and phosphorus?
SAMPLE EXERCISE 21.4 Predicting Nuclear Stability
Predict which of these nuclei are especially stable: ![]() ,
, ![]() ,
, ![]() .
.
SOLUTION
Analyze We are asked to identify especially stable nuclei, given their mass numbers and atomic numbers.
Plan We look to see whether the numbers of protons and neutrons correspond to magic numbers.
Solve The ![]() nucleus (the alpha particle) has a magic number of both protons (2) and neutrons (2) and is very stable. The
nucleus (the alpha particle) has a magic number of both protons (2) and neutrons (2) and is very stable. The ![]() nucleus also has a magic number of both protons (20) and neutrons (20) and is especially stable.
nucleus also has a magic number of both protons (20) and neutrons (20) and is especially stable.
The ![]() nucleus does not have a magic number of either protons or neutrons. In fact, it has an odd number of both protons (43) and neutrons (55). There are very few stable nuclei with odd numbers of both protons and neutrons. Indeed, technetium-98 is radioactive.
nucleus does not have a magic number of either protons or neutrons. In fact, it has an odd number of both protons (43) and neutrons (55). There are very few stable nuclei with odd numbers of both protons and neutrons. Indeed, technetium-98 is radioactive.
PRACTICE EXERCISE
Which of the following nuclei would you expect to exhibit a special stability: ![]() ,
, ![]() ,
, ![]() ?
?
Answer: ![]() ,
, ![]()