CHEMISTRY THE CENTRAL SCIENCE
21 NUCLEAR CHEMISTRY
EXERCISES
VISUALIZING CONCEPTS
21.1 Indicate whether each of the following nuclides lies within the belt of stability in Figure 21.2: (a) neon-24, (b) chlorine-32, (c) tin-108, (d) polonium-216. For any that do not, describe a nuclear decay process that would alter the neutron-to-proton ratio in the direction of increased stability. [Section 21.2]
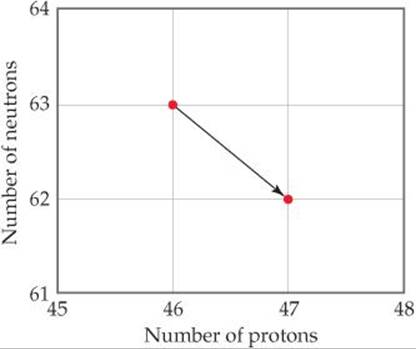
21.2 Write the balanced nuclear equation for the reaction represented by the diagram shown here. [Section 21.2]
21.3 Draw a diagram similar to that shown in Exercise 21.2 that illustrates the nuclear reaction ![]() . [Section 21.2]
. [Section 21.2]
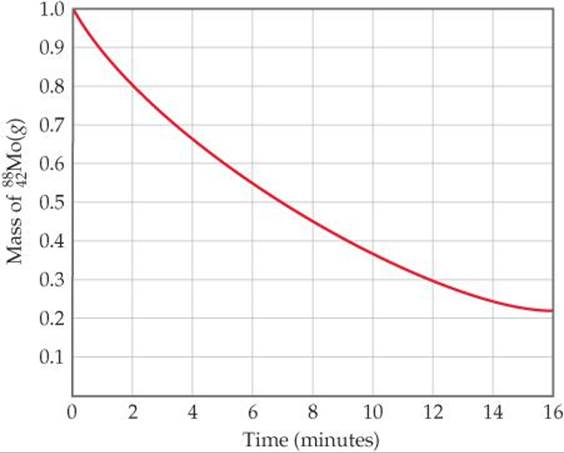
21.4 The accompanying graph illustrates the decay of ![]() , which decays via positron emission. (a) What is the half-life of the decay? (b) What is the rate constant for the decay? (c) What fraction of the original sample of
, which decays via positron emission. (a) What is the half-life of the decay? (b) What is the rate constant for the decay? (c) What fraction of the original sample of ![]() remains after 12 min? (d) What is the product of the decay process? [Section 21.4]
remains after 12 min? (d) What is the product of the decay process? [Section 21.4]
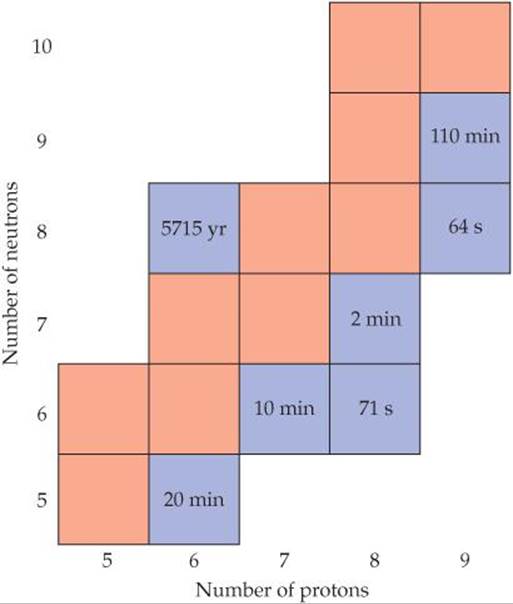
21.5 All the stable isotopes of boron, carbon, nitrogen, oxygen, and fluorine are shown in the chart in the right hand column (in red), along with their radioactive isotopes with t1/2> 1 min (in blue). (a) Write the chemical symbols, including mass and atomic numbers, for all of the stable isotopes. (b) Which radioactive isotopes are most likely to decay by beta emission? (c) Some of the isotopes shown are used in positron emission tomography. Which ones would you expect to be most useful for this application? (d) Which isotope would decay to 12.5% of its original concentration after 1 hour? [Sections 21.2, 21.4, and 21.5]
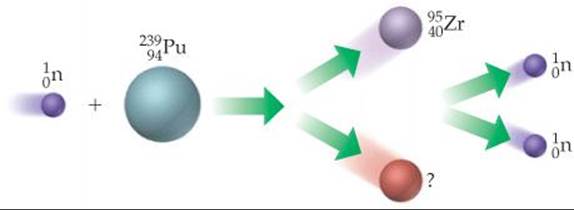
21.6 The diagram shown here illustrates a fission process. (a) What is the unidentified product of the fission? (b) Use Figure 21.2 to predict whether the nuclear products of this fission reaction are stable. [Section 21.7]
RADIOACTIVITY (section 21.1)
21.7 Indicate the number of protons and neutrons in the following nuclei: ![]() , (b) 201Hg, (c) potassium-39.
, (b) 201Hg, (c) potassium-39.
21.8 Indicate the number of protons and neutrons in the following nuclei: ![]() , (b) 37Cl, (c) thorium-232.
, (b) 37Cl, (c) thorium-232.
21.9 Give the symbol for (a) a neutron, (b) an alpha particle, (c) gamma radiation.
21.10 Give the symbol for (a) a proton, (b) a beta particle, (c) a positron.
21.11 Write balanced nuclear equations for the following processes: (a) rubidium-90 undergoes beta emission; (b) selenium-72 undergoes electron capture; (c) krypton-76 undergoes positron emission; (d) radium-226 emits alpha radiation.
21.12 Write balanced nuclear equations for the following transformations: (a) bismuth-213 undergoes alpha decay; (b) nitrogen-13 undergoes electron capture; (c) technicium-98 undergoes electron capture; (d) gold-188 decays by positron emission.
21.13 Decay of which nucleus will lead to the following products: (a) bismuth-211 by beta decay; (b) chromium-50 by positron emission; (c) tantalum-179 by electron capture; (d) radium-226 by alpha decay?
21.14 What particle is produced during the following decay processes: (a) sodium-24 decays to magnesium-24; (b) mercury-188 decays to gold-188; (c) iodine-122 decays to xenon-122; (d) plutonium-242 decays to uranium-238?
21.15 The naturally occurring radioactive decay series that begins with ![]() stops with formation of the stable
stops with formation of the stable ![]() nucleus. The decays proceed through a series of alpha-particle and beta-particle emissions. How many of each type of emission are involved in this series?
nucleus. The decays proceed through a series of alpha-particle and beta-particle emissions. How many of each type of emission are involved in this series?
21.16 A radioactive decay series that begins with ![]() ends with formation of the stable nuclide
ends with formation of the stable nuclide ![]() . How many alpha-particle emissions and how many beta-particle emissions are involved in the sequence of radioactive decays?
. How many alpha-particle emissions and how many beta-particle emissions are involved in the sequence of radioactive decays?
NUCLEAR STABILITY (section 21.2)
21.17 Predict the type of radioactive decay process for the following radionuclides: (a) ![]() , (b)
, (b) ![]() , (c) phosphorus-32, (d) chlorine-39.
, (c) phosphorus-32, (d) chlorine-39.
21.18 Each of the following nuclei undergoes either beta decay or positron emission. Predict the type of emission for each: (a) tritium, ![]() , (c) iodine-120, (d) silver-102.
, (c) iodine-120, (d) silver-102.
21.19 One of the nuclides in each of the following pairs is radioactive. Predict which is radioactive and which is stable: (a) ![]() , (b) 209Bi and 208Bi, (c) nickel-58 and nickel-65. Explain.
, (b) 209Bi and 208Bi, (c) nickel-58 and nickel-65. Explain.
21.20 One nuclide in each of these pairs is radioactive. Predict which is radioactive and which is stable: (a) ![]() , (b) 12C and 14C, (c) lead-206 and thorium-230. Explain your choice in each case.
, (b) 12C and 14C, (c) lead-206 and thorium-230. Explain your choice in each case.
21.21 Which of the following nuclides have magic numbers of both protons and neutrons: (a) helium-4, (b) oxygen-18, (c) calcium-40, (d) zinc-66, (e) lead-208?
21.22 Despite the similarities in the chemical reactivity of elements in the lanthanide series, their abundances in Earth's crust vary by two orders of magnitude. This graph shows the relative abundance as a function of atomic number. How do you explain the sawtooth variation across the series?
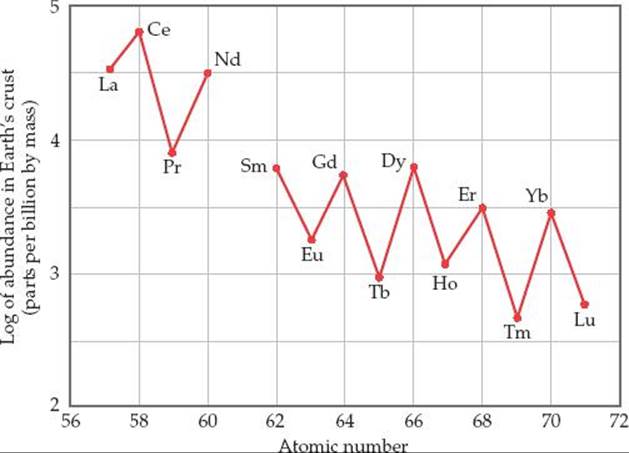
21.23 Using the concept of magic numbers, explain why alpha emission is relatively common, but proton emission is nonexistent.
21.24 Which of the following nuclides would you expect to be radioactive: ![]() , tungsten-184, polonium-206? Justify your choices.
, tungsten-184, polonium-206? Justify your choices.
NUCLEAR TRANSMUTATIONS (section 21.3)
21.25 Why are nuclear transmutations involving neutrons generally easier to accomplish than those involving protons or alpha particles?
21.26 In 1930 the American physicist Ernest Lawrence designed the first cyclotron in Berkeley, California. In 1937 Lawrence bombarded a molybdenum target with deuterium ions, producing for the first time an element not found in nature. What was this element? Starting with molybdenum-96 as your reactant, write a nuclear equation to represent this process.
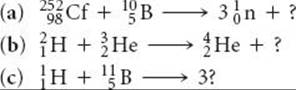
21.27 Complete and balance the following nuclear equations by supplying the missing particle:
![]()
21.28 Complete and balance the following nuclear equations by supplying the missing particle:
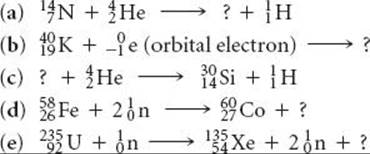
21.29 Write balanced equations for ![]()
![]() .
.
21.30 Write balanced equations for each of the following nuclear reactions: ![]() .
.
RATES OF RADIOACTIVE DECAY (section 21.4)
21.31 Each statement that follows refers to a comparison between two radioisotopes, A and X. Indicate whether each of the following statements is true or false, and why.
(a) If the half-life for A is shorter than the half-life for X, A has a larger decay rate constant.
(b) If X is “not radioactive,” its half-life is essentially zero.
(c) If A has a half-life of 10 years, and X has a half-life of 10,000 years, A would be a more suitable radioisotope to measure processes occurring on the 40-year time scale.
21.32 It has been suggested that strontium-90 (generated by nuclear testing) deposited in the hot desert will undergo radioactive decay more rapidly because it will be exposed to much higher average temperatures. (a) Is this a reasonable suggestion? (b) Does the process of radioactive decay have an activation energy, like the Arrhenius behavior of many chemical reactions (Section 14.5)? Discuss.
21.33 Some watch dials are coated with a phosphor, like ZnS, and a polymer in which some of the 1H atoms have been replaced by 3H atoms, tritium. The phosphor emits light when struck by the beta particle from the tritium decay, causing the dials to glow in the dark. The half-life of tritium is 12.3 yr. If the light given off is assumed to be directly proportional to the amount of tritium, by how much will a dial be dimmed in a watch that is 50 years old?
21.34 It takes 5.2 min for a 1.000-g sample of 210Fr to decay to 0.250 g. What is the half-life of 210Fr?
21.35 Cobalt-60 is a strong gamma emitter that has a half-life of 5.26 yr. The cobalt-60 in a radiotherapy unit must be replaced when its radioactivity falls to 75% of the original sample. If an original sample was purchased in June 2010, when will it be necessary to replace the cobalt-60?
21.36 How much time is required for a 6.25-mg sample of 51Cr to decay to 0.75 mg if it has a half-life of 27.8 days?
[21.37] Radium-226, which undergoes alpha decay, has a half-life of 1600 yr. (a) How many alpha particles are emitted in 5.0 min by a 10.0-mg sample of 226Ra? (b) What is the activity of the sample in mCi?
[21.38] Cobalt-60, which undergoes beta decay, has a half-life of 5.26 yr. (a) How many beta particles are emitted in 600 s by a 3.75-mg sample of 60Co? (b) What is the activity of the sample in Bq?
21.39 The cloth shroud from around a mummy is found to have a 14C activity of 9.7 disintegrations per minute per gram of carbon as compared with living organisms that undergo 16.3 disintegrations per minute per gram of carbon. From the half-life for 14C decay, 5715 yr, calculate the age of the shroud.
21.40 A wooden artifact from a Chinese temple has a 14C activity of 38.0 counts per minute as compared with an activity of 58.2 counts per minute for a standard of zero age. From the half-life for 14C decay, 5715 yr, determine the age of the artifact.
21.41 Potassium-40 decays to argon-40 with a half-life of 1.27 × 109 yr. What is the age of a rock in which the mass ratio of 40Ar to 40K is 4.2?
21.42 The half-life for the process ![]() is 4.5 × 109 yr. A mineral sample contains 75.0 mg of 238U and 18.0 mg of 206Pb. What is the age of the mineral?
is 4.5 × 109 yr. A mineral sample contains 75.0 mg of 238U and 18.0 mg of 206Pb. What is the age of the mineral?
ENERGY CHANGES (section 21.6)
21.43 The thermite reaction, ![]()
![]() kJ/mol, is one of the most exothermic reactions known. Because the heat released is sufficient to melt the iron product, the reaction is used to weld metal under the ocean. How much heat is released per mole of Fe2O3 produced? How does this amount of thermal energy compare with the energy released when 2 mol of protons and 2 mol of neutrons combine to form 1 mol of alpha particles?
kJ/mol, is one of the most exothermic reactions known. Because the heat released is sufficient to melt the iron product, the reaction is used to weld metal under the ocean. How much heat is released per mole of Fe2O3 produced? How does this amount of thermal energy compare with the energy released when 2 mol of protons and 2 mol of neutrons combine to form 1 mol of alpha particles?
21.44 An analytical laboratory balance typically measures mass to the nearest 0.1 mg. What energy change would accompany the loss of 0.1 mg in mass?
21.45 How much energy must be supplied to break a single aluminum-27 nucleus into separated protons and neutrons if an aluminum-27 atom has a mass of 26.9815386 amu? How much energy is required for 100.0 grams of aluminum-27? (The mass of an electron is given on the inside back cover.)
21.46 How much energy must be supplied to break a single 21Ne nucleus into separated protons and neutrons if the nucleus has a mass of 20.98846 amu? What is the nuclear binding energy for 1 mol of 21Ne?
21.47 The atomic masses of hydrogen-2 (deuterium), helium-4, and lithium-6 are 2.014102 amu, 4.002602 amu, and 6.0151228 amu, respectively. For each isotope, calculate (a) the nuclear mass, (b) the nuclear binding energy, (c) the nuclear binding energy per nucleon. (d) Which of these three isotopes has the largest nuclear binding energy per nucleon? Does this agree with the trends plotted in Figure 21.12?
21.48 The atomic masses of nitrogen-14, titanium-48, and xenon-129 are 13.999234 amu, 47.935878 amu, and 128.904779 amu, respectively. For each isotope, calculate (a) the nuclear mass, (b) the nuclear binding energy, (c) the nuclear binding energy per nucleon.
21.49 The energy from solar radiation falling on Earth is 1.07 × 1016 kJ/min. (a) How much loss of mass from the Sun occurs in one day from just the energy falling on Earth? (b) If the energy released in the reaction
![]()
(235U nuclear mass, 234.9935 amu; 141Ba nuclear mass, 140.8833 amu; 92Kr nuclear mass, 91.9021 amu) is taken as typical of that occurring in a nuclear reactor, what mass of uranium-235 is required to equal 0.10% of the solar energy that falls on Earth in 1.0 day?
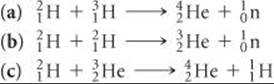
21.50 Based on the following atomic mass values—1H, 1.00782 amu; 2H, 2.01410 amu; 3H, 3.01605 amu; 3He, 3.01603 amu; 4He, 4.00260 amu—and the mass of the neutron given in the text, calculate the energy released per mole in each of the following nuclear reactions, all of which are possibilities for a controlled fusion process:
21.51 Which of the following nuclei is likely to have the largest mass defect per nucleon: (a) 59Co, (b) 11B, (c) 118Sn, (d) 243Cm? Explain your answer.
21.52 The isotope ![]() has the largest binding energy per nucleon of any isotope. Calculate this value from the atomic mass of nickel-62 (61.928345 amu) and compare it with the value given for iron-56 in Table 21.7.
has the largest binding energy per nucleon of any isotope. Calculate this value from the atomic mass of nickel-62 (61.928345 amu) and compare it with the value given for iron-56 in Table 21.7.
EFFECTS AND USES OF RADIOISOTOPES (sections 21.7–21.9)
21.53 Iodine-131 is a convenient radioisotope to monitor thyroid activity in humans. It is a beta emitter with a half-life of 8.02 days. The thyroid is the only gland in the body that uses iodine. A person undergoing a test of thyroid activity drinks a solution of NaI, in which only a small fraction of the iodide is radioactive. (a) Why is NaI a good choice for the source of iodine? (b) If a Geiger counter is placed near the person's thyroid (which is near the neck) right after the sodium iodide solution is taken, what will the data look like as a function of time? (c) A normal thyroid will take up about 12% of the ingested iodide in a few hours. How long will it take for the radioactive iodide taken up and held by the thyroid to decay to 0.01% of the original amount?
21.54 Why is it important that radioisotopes used as diagnostic tools in nuclear medicine produce gamma radiation when they decay? Why are alpha emitters not used as diagnostic tools?
21.55 What is the most common fissionable isotope in a commercial nuclear power reactor?
21.56 What is meant by enriched uranium? How is enriched uranium different from natural uranium?
21.57 What is the function of the control rods in a nuclear reactor? What substances are used to construct control rods? Why are these substances chosen?
21.58 (a) What is the function of the moderator in a nuclear reactor? (b) What substance acts as the moderator in a pressurized water generator? (c) What other substances are used as a moderator in nuclear reactor designs?
21.59 Complete and balance the nuclear equations for the following fission or fusion reactions:
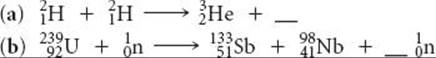
21.60 Complete and balance the nuclear equations for the following fission reactions:
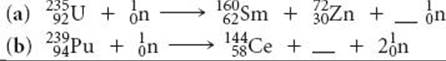
21.61 A portion of the Sun's energy comes from the reaction
![]()
which requires a temperature of 106 to 107 K. (a) Use the mass of the helium-4 nucleus given in Table 21.7 to determine how much energy is released when the reaction is run with 1 mol of hydrogen atoms. (b) Why is such a high temperature required?
21.62 The spent fuel elements from a fission reactor are much more intensely radioactive than the original fuel elements. (a) What does this tell you about the products of the fission process in relationship to the belt of stability, Figure 21.2? (b) Given that only two or three neutrons are released per fission event and knowing that the nucleus undergoing fission has a neutron-to-proton ratio characteristic of a heavy nucleus, what sorts of decay would you expect to be dominant among the fission products?
21.63 Which type or types of nuclear reactors have these characteristics?
(a) Does not use a secondary coolant
(b) Creates more fissionable material than it consumes
(c) Uses a gas, such as He or CO2, as the primary coolant
21.64 Which type or types of nuclear reactors have these characteristics?
(a) Can use natural uranium as a fuel
(b) Does not use a moderator
(s) Can be refueled without shutting down
21.65 Hydroxyl radicals can pluck hydrogen atoms from molecules (“hydrogen abstraction”), and hydroxide ions can pluck protons from molecules (“deprotonation”). Write the reaction equations and Lewis dot structures for the hydrogen abstraction and deprotonation reactions for the generic carboxylic acid R–COOH with hydroxyl radical and hydroxide ion, respectively. Why is hydroxyl radical more toxic to living systems than hydroxide ion?
21.66 Which are classified as ionizing radiation: X-rays, alpha particles, microwaves from a cell phone, and gamma rays?
21.67 A laboratory rat is exposed to an alpha-radiation source whose activity is 14.3 mCi. (a) What is the activity of the radiation in disintegrations per second? In becquerels? (b) The rat has a mass of 385 g and is exposed to the radiation for 14.0 s, absorbing 35% of the emitted alpha particles, each having an energy of 9.12 × 10–13 J. Calculate the absorbed dose in mil-lirads and grays. (c) If the RBE of the radiation is 9.5, calculate the effective absorbed dose in mrem and Sv.
21.68 A 65-kg person is accidentally exposed for 240 s to a 15-mCi source of beta radiation coming from a sample of 90Sr. (a) What is the activity of the radiation source in disintegrations per second? In becquerels? (b) Each beta particle has an energy of 8.75 × 10–14 J, and 7.5% of the radiation is absorbed by the person. Assuming that the absorbed radiation is spread over the person's entire body, calculate the absorbed dose in rads and in grays. (c) If the RBE of the beta particles is 1.0, what is the effective dose in mrem and in sieverts? (d) How does the magnitude of this dose of radiation compare with that of a mammogram (300 mrem)?
ADDITIONAL EXERCISES
21.69 Radon-222 decays to a stable nucleus by a series of three alpha emissions and two beta emissions. What is the stable nucleus that is formed?
21.70 Equation 21.28 is the nuclear reaction responsible for much of the helium-4 production in our Sun. How much energy is released in this reaction?
21.71 Chlorine has two stable nuclides, 35Cl and 37Cl. In contrast, 36Cl is a radioactive nuclide that decays by beta emission. (a) What is the product of decay of 36Cl? (b) Based on the empirical rules about nuclear stability, explain why the nucleus of 36Cl is less stable than either 35Cl or37Cl.
21.72 When two protons fuse in a star, the product is 2H plus a positron (Equation 21.26). Why do you think the more obvious product of the reaction, 2He, is unstable?
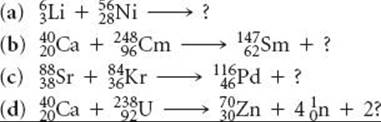
21.73 Nuclear scientists have synthesized approximately 1600 nuclei not known in nature. More might be discovered with heavy-ion bombardment using high-energy particle accelerators. Complete and balance the following reactions, which involve heavy-ion bombardments:
[21.74] The synthetic radioisotope technetium-99, which decays by beta emission, is the most widely used isotope in nuclear medicine. The following data were collected on a sample of 99Tc:
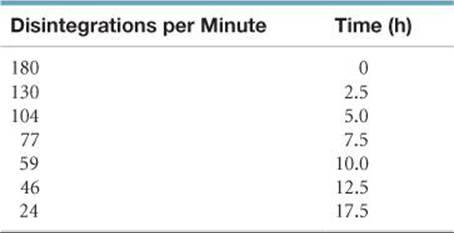
Using these data, make an appropriate graph and curve fit to determine the half-life.
[21.75] According to current regulations, the maximum permissible dose of strontium-90 in the body of an adult is 1μCi (1 × 10–6 Ci). Using the relationship rate = kN, calculate the number of atoms of strontium-90 to which this dose corresponds. To what mass of strontium-90 does this correspond? The half-life for strontium-90 is 28.8 yr.
[21.76] Suppose you had a detection device that could count every decay event from a radioactive sample of plutonium-239 (t1/2 is 24,000 yr). How many counts per second would you obtain from a sample containing 0.385 g of plutonium-239?
21.77 Methyl acetate (CH3COOCH3) is formed by the reaction of acetic acid with methyl alcohol. If the methyl alcohol is labeled with oxygen-18, the oxygen-18 ends up in the methyl acetate:

Do the C — OH bond of the acid and the O — H bond of the alcohol break in the reaction, or do the O — H bond of the acid and the C — OH bond of the alcohol break? Explain.
21.78 An experiment was designed to determine whether an aquatic plant absorbed iodide ion from water. Iodine-131 (t1/2 = 8.02 days) was added as a tracer, in the form of iodide ion, to a tank containing the plants. The initial activity of a 1.00-μL sample of the water was 214 counts per minute. After 30 days the level of activity in a 1.00-μL sample was 15.7 counts per minute. Did the plants absorb iodide from the water? Explain.
21.79 The nuclear masses of 7Be, 9Be, and 10Be are 7.0147, 9.0100, and 10.0113 amu, respectively. Which of these nuclei has the largest binding energy per nucleon?
[21.80] A 26.00-g sample of water containing tritium, ![]() , emits 1.50 × 103 beta particles per second. Tritium is a weak beta emitter with a half-life of 12.3 yr. What fraction of all the hydrogen in the water sample is tritium?
, emits 1.50 × 103 beta particles per second. Tritium is a weak beta emitter with a half-life of 12.3 yr. What fraction of all the hydrogen in the water sample is tritium?
21.81 The Sun radiates energy into space at the rate of 3.9 × 1026 J/s. (a) Calculate the rate of mass loss from the Sun in kg/s. (b) How does this mass loss arise? (c) It is estimated that the Sun contains 9 × 1056 free protons. How many protons per second are consumed in nuclear reactions in the Sun?
[21.82] The average energy released in the fission of a single ura-nium-235 nucleus is about 3 × 10–11 J. If the conversion of this energy to electricity in a nuclear power plant is 40% efficient, what mass of uranium-235 undergoes fission in a year in a plant that produces 1000 megawatts? Recall that a watt is 1 J/s.
21.83 Tests on human subjects in Boston in 1965 and 1966, following the era of atomic bomb testing, revealed average quantities of about 2 pCi of plutonium radioactivity in the average person. How many disintegrations per second does this level of activity imply? If each alpha particle deposits 8 × 10–13 J of energy and if the average person weighs 75 kg, calculate the number of rads and rems of radiation in 1 yr from such a level of plutonium.
INTEGRATIVE EXERCISES
21.84 A 53.8-mg sample of sodium perchlorate contains radioactive chlorine-36 (whose atomic mass is 36.0 amu). If 29.6% of the chlorine atoms in the sample are chlorine-36 and the remainder are naturally occurring nonradioactive chlorine atoms, how many disintegrations per second are produced by this sample? The half-life of chlorine-36 is 3.0 × 105 yr.
21.85 Calculate the mass of octane, C8H18(l), that must be burned in air to evolve the same quantity of energy as produced by the fusion of 1.0 g of hydrogen in the following fusion reaction:
![]()
Assume that all the products of the combustion of octane are in their gas phases. Use data from Exercise 21.50, Appendix C, and the inside covers of the text. The standard enthalpy of formation of octane is —250.1 kJ/mol.
21.86 A sample of an alpha emitter having an activity of 0.18i is stored in a 25.0-mL sealed container at 22 °C for 245 days. (a) How many alpha particles are formed during this time? (b) Assuming that each alpha particle is converted to a helium atom, what is the partial pressure of helium gas in the container after this 245-day period?
[21.87] Charcoal samples from Stonehenge in England were burned in O2, and the resultant CO2 gas bubbled into a solution of Ca(OH)2 (limewater), resulting in the precipitation of CaCO3. The CaCO3 was removed by filtration and dried. A 788-mg sample of the CaCO3 had a radioactivity of 1.5 × 10–2 Bq due to carbon-14. By comparison, living organisms undergo 15.3 disintegrations per minute per gram of carbon. Using the half-life of carbon-14, 5715 yr, calculate the age of the charcoal sample.
21.88 A 25.0-mL sample of 0.050 M barium nitrate solution was mixed with 25.0 mL of 0.050 M sodium sulfate solution labeled with radioactive sulfur-35. The activity of the initial sodium sulfate solution was 1.22 × 106 Bq/mL. After the resultant precipitate was removed by filtration, the remaining filtrate was found to have an activity of 250 Bq/mL. (a) Write a balanced chemical equation for the reaction that occurred. (b) Calculate the Ksp for the precipitate under the conditions of the experiment.