CHEMISTRY THE CENTRAL SCIENCE
22 CHEMISTRY OF THE NONMETALS
22.5 OXYGEN
By the middle of the seventeenth century, scientists recognized that air contained a component associated with burning and breathing. That component was not isolated until 1774, however, when English scientist Joseph Priestley discovered oxygen. Lavoisier subsequently named the elementoxygen, meaning “acid former.” There is some historical debate about who “really” discovered oxygen, and the debate is the subject of a play written by the chemist-authors Carl Djerassi and Roald Hoffmann (![]() FIGURE 22.12).
FIGURE 22.12).

![]() FIGURE 22.12 Oxygen is the name of a play by the chemists Carl Djerassi (Stanford University) and Roald Hoffmann (Cornell University). Its subject is the controversy over who discovered oxygen.
FIGURE 22.12 Oxygen is the name of a play by the chemists Carl Djerassi (Stanford University) and Roald Hoffmann (Cornell University). Its subject is the controversy over who discovered oxygen.
Oxygen is found in combination with other elements in a great variety of compounds—water (H2O), silica (SiO2), alumina (Al2O3), and the iron oxides (Fe2O3, Fe3O4) are obvious examples. Indeed, oxygen is the most abundant element by mass both in Earth's crust and in the human body. ![]() (Section 1.2) It is the oxidizing agent for the metabolism of our foods and is crucial to human life.
(Section 1.2) It is the oxidizing agent for the metabolism of our foods and is crucial to human life.
Properties of Oxygen
Oxygen has two allotropes, O2 and O3. When we speak of molecular oxygen or simply oxygen, it is usually understood that we are speaking of dioxygen (O2), the normal form of the element; O3 is ozone.
At room temperature, dioxygen is a colorless, odorless gas. It condenses to a liquid at – 183 °C and freezes at –218 °C. It is only slightly soluble in water (0.04 g/L, or 0.001 M at 25 °C), but its presence in water is essential to marine life.
The electron configuration of the oxygen atom is [He]2s 22p4. Thus, oxygen can complete its octet of valence electrons either by picking up two electrons to form the oxide ion (O2–) or by sharing two electrons. In its covalent compounds, it tends to form either two single bonds, as in H2O, or a double bond, as in formaldehyde (H2C ═ O). The O2 molecule contains a double bond. The bond in O2 is very strong (bond enthalpy 495 kJ/mol). Oxygen also forms strong bonds with many other elements. Consequently, many oxygen-containing compounds are thermodynamically more stable than O2. In the absence of a catalyst, however, most reactions of O2 have high activation energies and thus require high temperatures to proceed at a suitable rate. Once a sufficiently exothermic reaction begins, it may accelerate rapidly, producing a reaction of explosive violence.
Production of Oxygen
Nearly all commercial oxygen is obtained from air. The normal boiling point of O2 is –183 °C, whereas that of N2, the other principal component of air, is –196 °C. Thus, when air is liquefied and then allowed to warm, the N2 boils off, leaving liquid O2 contaminated mainly by small amounts of N2 and Ar.
In the laboratory, O2 can be obtained by heating either aqueous hydrogen peroxide or solid potassium chlorate (KClO3):
![]()
Manganese dioxide (MnO2) catalyzes both reactions.
Much of the O2 in the atmosphere is replenished through photosynthesis, in which green plants use the energy of sunlight to generate O2 (along with glucose, C6H12O6) from atmospheric CO2:
![]()
Uses of Oxygen
In industrial use, oxygen ranks behind only sulfuric acid (H2SO4) and nitrogen (N2). About 3 × 1010 kg (30 million tons) of O2 is used annually in the United States. It is shipped and stored either as a liquid or in steel containers as a compressed gas. About 70% of the O2 output, however, is generated where it is needed.
Oxygen is by far the most widely used oxidizing agent in industry. Over half of the O2 produced is used in the steel industry, mainly to remove impurities from steel. It is also used to bleach pulp and paper. (Oxidation of colored compounds often gives colorless products.) Oxygen is used together with acetylene (C2H2) in oxyacetylene welding (![]() FIGURE 22.13). The reaction between C2H2 and O2 is highly exothermic, producing temperatures in excess of 3000 °C.
FIGURE 22.13). The reaction between C2H2 and O2 is highly exothermic, producing temperatures in excess of 3000 °C.

![]() FIGURE 22.13 Welding with an oxyacetylene torch.
FIGURE 22.13 Welding with an oxyacetylene torch.
Ozone
Ozone is a pale blue, poisonous gas with a sharp, irritating odor. Most people can detect about 0.01 ppm in air. Exposure to 0.1 to 1 ppm produces headaches, burning eyes, and irritation to the respiratory passages.
The O3 molecule possesses a π bond that is delocalized over the three oxygen atoms. ![]() (Section 8.6) The molecule dissociates readily, forming reactive oxygen atoms:
(Section 8.6) The molecule dissociates readily, forming reactive oxygen atoms:
![]()
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
What wavelength of light is needed to break an O—O bond in ozone?
Ozone is a stronger oxidizing agent than dioxygen. Ozone forms oxides with many elements under conditions where O2 will not react; indeed, it oxidizes all the common metals except gold and platinum.
Ozone can be prepared by passing electricity through dry O2 in a flow-through apparatus. The electrical discharge causes the O2 bond to break, resulting in reactions like those described in Section 18.1. During thunderstorms, ozone is generated (and can be smelled, if you are too close) from lightning strikes:
![]()
Ozone cannot be stored for long, except at low temperature, because it readily decomposes to O2. The decomposition is catalyzed by Ag, Pt, and Pd and by many transition-metal oxides.
SAMPLE EXERCISE 22.6 Calculating an Equilibrium Constant
Using ![]() for ozone from Appendix C, calculate the equilibrium constant for Equation 22.24 at 298.0 K, assuming no electrical input.
for ozone from Appendix C, calculate the equilibrium constant for Equation 22.24 at 298.0 K, assuming no electrical input.
SOLUTION
Analyze We are asked to calculate the equilibrium constant for the formation of O3 from O2, given the temperature and![]() .
.
Plan The relationship between the standard free-energy change, ![]() , for a reaction and the equilibrium constant for the reaction is given in Equation 19.20.
, for a reaction and the equilibrium constant for the reaction is given in Equation 19.20.
Solve From Appendix C we have ![]()
Thus, for Equation 22.24,![]()
From Equation 19.20 we have ![]()
Thus, 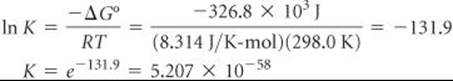
Comment In spite of the unfavorable equilibrium constant, ozone can be prepared from O2 as described in the preceding text. The unfavorable free energy of formation is overcome by energy from the electrical discharge, and O3 is removed before the reverse reaction can occur, so a nonequilibrium mixture results.
PRACTICE EXERCISE
Using the data in Appendix C, calculate ΔG° and K for Equation 22.23 at 298.0 K.
Answer: ΔG° = 66.7 kJ, K = 2.03 × 10–12
Ozone is sometimes used to treat drinking water. Like Cl2, ozone kills bacteria and oxidizes organic compounds. The largest use of ozone, however, is in the preparation of pharmaceuticals, synthetic lubricants, and other commercially useful organic compounds, where O3 is used to sever carbon–carbon double bonds.
Ozone is an important component of the upper atmosphere, where it screens out ultraviolet radiation and so protects us from the effects of these high-energy rays. For this reason, depletion of stratospheric ozone is a major scientific concern. ![]() (Section 18.2) In the lower atmosphere, ozone is considered an air pollutant and is a major constituent of smog.
(Section 18.2) In the lower atmosphere, ozone is considered an air pollutant and is a major constituent of smog. ![]() (Section 18.2) Because of its oxidizing power, ozone damages living systems and structural materials, especially rubber.
(Section 18.2) Because of its oxidizing power, ozone damages living systems and structural materials, especially rubber.
Oxides
The electronegativity of oxygen is second only to that of fluorine. As a result, oxygen has negative oxidation states in all compounds except OF2 and O2F2. The –2 oxidation state is by far the most common. Compounds in this oxidation state are called oxides.
Nonmetals form covalent oxides, most of which are simple molecules with low melting and boiling points. SiO2 and B2O3, however, have extended structures. Most nonmetal oxides combine with water to give oxyacids. Sulfur dioxide (SO2), for example, dissolves in water to give sulfurous acid (H2SO3):
![]()
This reaction and that of SO3 with H2O to form H2SO4 are largely responsible for acid rain. ![]() (Section 18.2) The analogous reaction of CO2 with H2O to form carbonic acid (H2CO3) causes the acidity of carbonated water.
(Section 18.2) The analogous reaction of CO2 with H2O to form carbonic acid (H2CO3) causes the acidity of carbonated water.
Oxides that form acids when they react with water are called either acidic anhydrides (anhydride means “without water”) or acidic oxides. A few nonmetal oxides, especially ones with the nonmetal in a low oxidation state—such as N2O, NO, and CO—do not react with water and are not acidic anhydrides.
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
What acid is produced by the reaction of I2O5 with water?
Most metal oxides are ionic compounds. The ionic oxides that dissolve in water form hydroxides and, consequently, are called either basic anhydrides or basic oxides. Barium oxide, for example, reacts with water to form barium hydroxide (![]() FIGURE 22.14). These kinds of reactions are due to the high basicity of the O2– ion and its virtually complete hydrolysis in water:
FIGURE 22.14). These kinds of reactions are due to the high basicity of the O2– ion and its virtually complete hydrolysis in water:
![]()
Even those ionic oxides that are insoluble in water tend to dissolve in strong acids. Iron(III) oxide, for example, dissolves in acids:
![]()
This reaction is used to remove rust (Fe2O3.nH2O) from iron or steel before a protective coat of zinc or tin is applied.
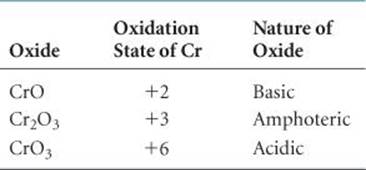
Oxides that can exhibit both acidic and basic character are said to be amphoteric. ![]() (Section 17.5) If a metal forms more than one oxide, the basic character of the oxide decreases as the oxidation state of the metal increases (
(Section 17.5) If a metal forms more than one oxide, the basic character of the oxide decreases as the oxidation state of the metal increases (![]() TABLE 22.4).
TABLE 22.4).
TABLE 22.4 • Acid–Base Character of Chromium Oxides
![]() GO FIGURE
GO FIGURE
Is this reaction a redox reaction?

![]() FIGURE 22.14 Reaction of a basic oxide with water.
FIGURE 22.14 Reaction of a basic oxide with water.
Peroxides and Superoxides
Compounds containing O — O bonds and oxygen in the –1 oxidation state are peroxides. Oxygen has an oxidation state of ![]() in O2–, which is called the superoxide ion. The most active (easily oxidized) metals (K, Rb, and Cs) react with O2 to give superoxides (KO2, RbO2, and CsO2). Their active neighbors in the periodic table (Na, Ca, Sr, and Ba) react with O2, producing peroxides (Na2O2, CaO2, SrO2, and BaO2). Less active metals and nonmetals produce normal oxides.
in O2–, which is called the superoxide ion. The most active (easily oxidized) metals (K, Rb, and Cs) react with O2 to give superoxides (KO2, RbO2, and CsO2). Their active neighbors in the periodic table (Na, Ca, Sr, and Ba) react with O2, producing peroxides (Na2O2, CaO2, SrO2, and BaO2). Less active metals and nonmetals produce normal oxides. ![]() (Section 7.6)
(Section 7.6)
When superoxides dissolve in water, O2 is produced:
![]()
Because of this reaction, potassium superoxide is used as an oxygen source in masks worn by rescue workers (![]() FIGURE 22.15). For proper breathing in toxic environments, oxygen must be generated in the mask and exhaled carbon dioxide in the mask must be eliminated. Moisture in the breath causes the KO2 to decompose to O2 and KOH, and the KOH removes CO2 from the exhaled breath:
FIGURE 22.15). For proper breathing in toxic environments, oxygen must be generated in the mask and exhaled carbon dioxide in the mask must be eliminated. Moisture in the breath causes the KO2 to decompose to O2 and KOH, and the KOH removes CO2 from the exhaled breath:
![]()

![]() FIGURE 22.15 A self-contained breathing apparatus.
FIGURE 22.15 A self-contained breathing apparatus.
Hydrogen peroxide (![]() FIGURE 22.16) is the most familiar and commercially important peroxide. Pure hydrogen peroxide is a clear, syrupy liquid that melts at —0.4 °C. Concentrated hydrogen peroxide is dangerously reactive because the decomposition to water and oxygen is very exothermic:
FIGURE 22.16) is the most familiar and commercially important peroxide. Pure hydrogen peroxide is a clear, syrupy liquid that melts at —0.4 °C. Concentrated hydrogen peroxide is dangerously reactive because the decomposition to water and oxygen is very exothermic:
![]()
![]() GO FIGURE
GO FIGURE
Does H2O2 have a dipole moment?
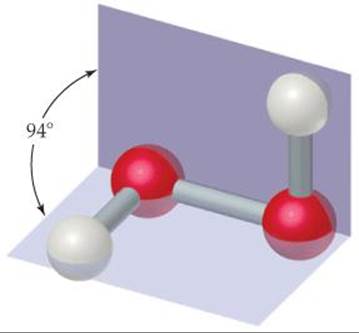
![]() FIGURE 22.16 Molecular structure of hydrogen peroxide.
FIGURE 22.16 Molecular structure of hydrogen peroxide.
This is another example of a disproportionation reaction, in which an element is simultaneously oxidized and reduced. The oxidation number of oxygen changes from –1 to –2 and 0.
Hydrogen peroxide is marketed as a chemical reagent in aqueous solutions of up to about 30% by mass. A solution containing about 3% H2O2 by mass is sold in drugstores and used as a mild antiseptic. Somewhat more concentrated solutions are used to bleach fabrics.
The peroxide ion is a by-product of metabolism that results from the reduction of O2. The body disposes of this reactive ion with enzymes such as peroxidase and catalase.