CHEMISTRY THE CENTRAL SCIENCE
22 CHEMISTRY OF THE NONMETALS
22.6 THE OTHER GROUP 6A ELEMENTS: S, Se, Te, AN D Po
The other group 6A elements are sulfur, selenium, tellurium, and polonium. In this section we will survey the properties of the group as a whole and then examine the chemistry of sulfur, selenium, and tellurium. We will not say much about polonium, which has no stable isotopes and is found only in minute quantities in radium-containing minerals.
General Characteristics of the Group 6A Elements
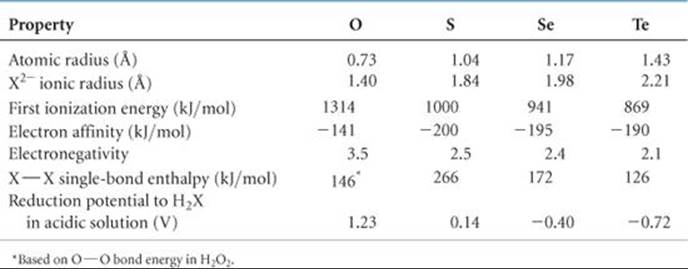
The group 6A elements possess the general outer-electron configuration ns2np4 with n ranging from 2 to 6. Thus, these elements attain a noble-gas electron configuration by adding two electrons, which results in a –2 oxidation state. Except for oxygen, the group 6A elements are also commonly found in positive oxidation states up to +6, and they can have expanded valence shells. Thus, we have such compounds as SF6, SeF6, and TeF6 with the central atom in the +6 oxidation state.
![]() TABLE 22.5 summarizes some properties of the group 6A elements.
TABLE 22.5 summarizes some properties of the group 6A elements.
Occurrence and Production of S, Se, and Te
Sulfur, selenium, and tellurium can all be mined from the earth. Large underground deposits are the principal source of elemental sulfur (![]() FIGURE 22.17). Sulfur also occurs widely as sulfide (S2–) and sulfate (SO42–) minerals. Its presence as a minor component of coal and petroleum poses a major problem. Combustion of these “unclean” fuels leads to serious pollution by sulfur oxides.
FIGURE 22.17). Sulfur also occurs widely as sulfide (S2–) and sulfate (SO42–) minerals. Its presence as a minor component of coal and petroleum poses a major problem. Combustion of these “unclean” fuels leads to serious pollution by sulfur oxides. ![]() (Section 18.2) Much effort has been directed at removing this sulfur, and these efforts have increased the availability of sulfur. The sale of this sulfur helps to partially offset the costs of the desulfurizing processes and equipment.
(Section 18.2) Much effort has been directed at removing this sulfur, and these efforts have increased the availability of sulfur. The sale of this sulfur helps to partially offset the costs of the desulfurizing processes and equipment.
![]() FIGURE 22.17 Massive amounts of sulfur are extracted every year from the earth.
FIGURE 22.17 Massive amounts of sulfur are extracted every year from the earth.
Selenium and tellurium occur in rare minerals, such as Cu2Se, PbSe, Cu2Te, and PbTe, and as minor constituents in sulfide ores of copper, iron, nickel, and lead.
Properties and Uses of Sulfur, Selenium, and Tellurium
Elemental sulfur is yellow, tasteless, and nearly odorless. It is insoluble in water and exists in several allotropic forms. The thermodynamically stable form at room temperature is rhombic sulfur, which consists of puckered S8 rings. ![]() (Figure 7.26) When heated above its melting point (113 °C), molten sulfur first contains S8 molecules and is fluid because the rings slip over one another. Further heating causes the rings to break; the fragments then join to form long molecules that can become entangled. In this polymeric form, sulfur becomes highly viscous. Further heating breaks the chains, and the viscosity again decreases.
(Figure 7.26) When heated above its melting point (113 °C), molten sulfur first contains S8 molecules and is fluid because the rings slip over one another. Further heating causes the rings to break; the fragments then join to form long molecules that can become entangled. In this polymeric form, sulfur becomes highly viscous. Further heating breaks the chains, and the viscosity again decreases.
Most of the approximately 1 × 1010 kg (10 million tons) of sulfur produced in the United States each year is used to manufacture sulfuric acid. Sulfur is also used to vulcanize rubber, a process that toughens rubber by introducing cross-linking between polymer chains. ![]() (Section 12.8)
(Section 12.8)
TABLE 22.5 • Some Properties of the Group 6A Elements
Selenium and tellurium do not form eight-membered rings in their elemental forms. ![]() (Section 7.8) The most stable allotropes of these elements are crystalline substances containing helical chains of atoms (
(Section 7.8) The most stable allotropes of these elements are crystalline substances containing helical chains of atoms (![]() FIGURE 22.18). Each atom is close to atoms in adjacent chains, and it appears that some sharing of electron pairs between these atoms occurs.
FIGURE 22.18). Each atom is close to atoms in adjacent chains, and it appears that some sharing of electron pairs between these atoms occurs.
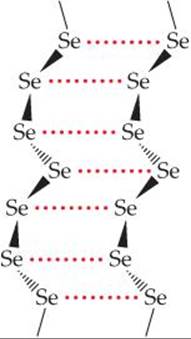
![]() FIGURE 22.18 Portion of helical chains making up the structure of crystalline selenium.
FIGURE 22.18 Portion of helical chains making up the structure of crystalline selenium.
The electrical conductivity of elemental selenium is low in the dark but increases greatly upon exposure to light. This property is exploited in photoelectric cells and light meters. Photocopiers also depend on the photoconductivity of selenium. Photocopy machines contain a belt or drum coated with a film of selenium. This drum is electrostatically charged and then exposed to light reflected from the image being photocopied. The charge drains from the regions where the selenium film has been made conductive by exposure to light. A black powder (the toner) sticks only to the areas that remain charged. The photocopy is made when the toner is transferred to a sheet of plain paper.
Sulfides
When an element is less electronegative than sulfur, sulfides that contain S 2– form. Many metallic elements are found in the form of sulfide ores, such as PbS (galena) and HgS (cinnabar). A series of related ores containing the disulfide ion, S22– (analogous to the peroxide ion), are known aspyrites. Iron pyrite, FeS2, occurs as golden yellow cubic crystals (![]() FIGURE 22.19). Because it has been occasionally mistaken for gold by miners, iron pyrite is often called fool's gold.
FIGURE 22.19). Because it has been occasionally mistaken for gold by miners, iron pyrite is often called fool's gold.

![]() FIGURE 22.19 Iron pyrite (FeS2, on the right) with gold for comparison.
FIGURE 22.19 Iron pyrite (FeS2, on the right) with gold for comparison.
One of the most important sulfides is hydrogen sulfide (H2S). This substance is not normally produced by direct union of the elements because it decomposes at elevated temperatures. It is normally prepared by action of dilute acid on iron(II) sulfide:
![]()
One of hydrogen sulfide's most readily recognized properties is its odor, which is most frequently encountered as the offensive odor of rotten eggs. Hydrogen sulfide is toxic but our noses can detect H2S in extremely low, nontoxic concentrations. A sulfur-containing organic molecule, such as dimethyl sulfide, (CH3)2S, which is similarly odoriferous and can be detected by smell at a level of one part per trillion, is added to natural gas as a safety factor to give it a detectable odor.
Oxides, Oxyacids, and Oxyanions of Sulfur
Sulfur dioxide, formed when sulfur burns in air, has a choking odor and is poisonous. The gas is particularly toxic to lower organisms, such as fungi, so it is used to sterilize dried fruit and wine. At 1 atm and room temperature, SO2 dissolves in water to produce a 1.6 M solution. The SO2solution is acidic, and we describe it as sulfurous acid (H2SO3).
Salts of SO32– (sulfites) and HSO3– (hydrogen sulfites or bisulfites) are well known. Small quantities of Na2SO3 or NaHSO3 are used as food additives to prevent bacterial spoilage. Because some people are extremely allergic to sulfites, all food products with sulfites must now carry a warning label disclosing their presence.
Although combustion of sulfur in air produces mainly SO2, small amounts of SO3 are also formed. The reaction produces chiefly SO2 because the activation-energy barrier for oxidation to SO3 is very high unless the reaction is catalyzed. Interestingly, the SO3 by-product is used industrially to make H2SO4, which is the ultimate product of the reaction between SO3 and water. In the manufacture of sulfuric acid, SO2 is obtained by burning sulfur and then oxidized to SO3, using a catalyst such as V2O5 or platinum. The SO3 is dissolved in H2SO4 because it does not dissolve quickly in water, and then the H2S2O7 formed in this reaction, called pyrosulfuric acid, is added to water to form H2SO4:
![]()
![]()
![]() GO FIGURE
GO FIGURE
In this reaction, what has happened to the H and O atoms in the sucrose?

![]() FIGURE 22.20 Sulfuric acid dehydrates table sugar to produce elemental carbon.
FIGURE 22.20 Sulfuric acid dehydrates table sugar to produce elemental carbon.
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
What is the net reaction of Equations 22.32 and 22.33?
Commercial sulfuric acid is 98% H2SO4. It is a dense, colorless, oily liquid that boils at 340 °C. It is a strong acid, a good dehydrating agent (![]() FIGURE 22.20), and a moderately good oxidizing agent.
FIGURE 22.20), and a moderately good oxidizing agent.
Year after year, the production of sulfuric acid is the largest of any chemical produced in the United States. About 4× 1010 kg (40 million tons) is produced annually in this country. Sulfuric acid is employed in some way in almost all manufacturing. Consequently, its consumption is considered a measure of industrial activity.
Sulfuric acid is a strong acid, but only the first hydrogen is completely ionized in aqueous solution:
![]()
![]()
Consequently, sulfuric acid forms both sulfates (SO42– salts) and bisulfates (or hydrogen sulfates, HSO4– salts). Bisulfate salts are common components of the “dry acids” used for adjusting the pH of swimming pools and hot tubs; they are also components of many toilet bowl cleaners.
The term thio indicates substitution of sulfur for oxygen, and the thiosulfate ion (S2O32–) is formed by boiling an alkaline solution of SO32– with elemental sulfur:
![]()
The structures of the sulfate and thiosulfate ions are compared in ![]() FIGURE 22.21. Thiosulfate salts are used industrially in the paper-making and textile industries and are the “fixer” solutions in the development of photographic film.
FIGURE 22.21. Thiosulfate salts are used industrially in the paper-making and textile industries and are the “fixer” solutions in the development of photographic film.
![]() GO FIGURE
GO FIGURE
What are the oxidation states of the sulfur atoms in the S2O32– ion?
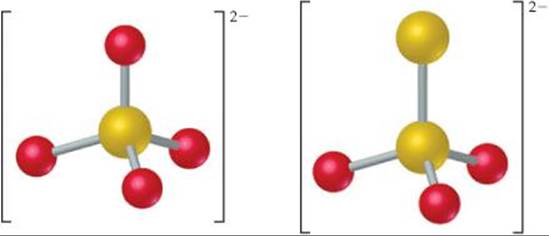
![]() FIGURE 22.21 Structures of the sulfate (left) and thiosulfate (right) ions.
FIGURE 22.21 Structures of the sulfate (left) and thiosulfate (right) ions.