CHEMISTRY THE CENTRAL SCIENCE
23 TRANSITION METALS AND COORDINATION CHEMISTRY

THIS STAINED GLASS WINDOW, representing chemistry, is in the Kalamazoo Public Library, Kalamazoo, Michigan.
WHAT'S AHEAD
23.1 THE TRANSITION METALS
We examine the physical properties, electron configurations, oxidation states, and magnetic properties of the transition metals.
23.2 TRANSITION-METAL COMPLEXES
We introduce the concepts of metal complexes and ligands and provide a brief history of the development of coordination chemistry.
23.3 COMMON LIGANDS IN COORDINATION CHEMISTRY
We examine some common geometries found in coordination complexes and how the geometries relate to coordination numbers.
23.4 NOMENCLATURE AND ISOMERISM IN COORDINATION CHEMISTRY
We introduce the nomenclature used for coordination compounds. We see that coordination compounds exhibit isomerism, in which two compounds have the same composition but different structures, and then look at two types: structural isomers and stereoisomers.
23.5 COLOR AND MAGNETISM IN COORDINATION CHEMISTRY
We discuss color and magnetism in coordination compounds, emphasizing the visible portion of the electromagnetic spectrum and the notion of complementary colors. We then see that many transition-metal complexes are paramagnetic because they contain unpaired electrons.
23.6 CRYSTAL-FIELD THEORY
We explore how crystal-field theory allows us to explain some of the interesting spectral and magnetic properties of coordination compounds.
THE COLORS OF OUR WORLD ARE beautiful, but to a chemist they are also informative—providing insights into the structure and bonding of matter. Compounds of the transition metals constitute an important group of colored substances. Some of them are used in paint pigments; others produce the colors in glass and precious gems. For example, the colors in the stained-glass artwork shown in the chapter-opening photograph are due mainly to transition-metal compounds. Why do these compounds have color, and why do the colors change as the ions or molecules bonded to the metal change? The chemistry we explore in this chapter will help us to answer these questions.
In earlier chapters we saw that metal ions can function as Lewis acids, forming covalent bonds with molecules and ions functioning as Lewis bases. ![]() (Section 16.11) We have encountered many ions and compounds that result from such interactions, such as [Fe(H2O)6]3+ and [Ag(NH3)2]+ in Sections 16.11 and 17.5 and hemoglobin in Section 13.6. In this chapter, we focus on the rich and important chemistry associated with such complex assemblies of metal ions surrounded by molecules and ions. Metal compounds of this kind are called coordination compounds, and the branch of chemistry that focuses on them is called coordination chemistry.
(Section 16.11) We have encountered many ions and compounds that result from such interactions, such as [Fe(H2O)6]3+ and [Ag(NH3)2]+ in Sections 16.11 and 17.5 and hemoglobin in Section 13.6. In this chapter, we focus on the rich and important chemistry associated with such complex assemblies of metal ions surrounded by molecules and ions. Metal compounds of this kind are called coordination compounds, and the branch of chemistry that focuses on them is called coordination chemistry.
23.1 THE TRANSITION METALS
The part of the periodic table in which the d orbitals are being filled as we move left to right across a row is the home of the transition metals (![]() FIGURE 23.1).
FIGURE 23.1). ![]() (Section 6.8) With some exceptions (e.g., platinum, gold), metallic elements are found in nature as solid inorganic compounds called minerals. Notice from
(Section 6.8) With some exceptions (e.g., platinum, gold), metallic elements are found in nature as solid inorganic compounds called minerals. Notice from ![]() TABLE 23.1 that minerals are identified by common names rather than chemical names. These common names are usually based on the location where a mineral was discovered, the person who discovered it, or some physical characteristic such as color.
TABLE 23.1 that minerals are identified by common names rather than chemical names. These common names are usually based on the location where a mineral was discovered, the person who discovered it, or some physical characteristic such as color.
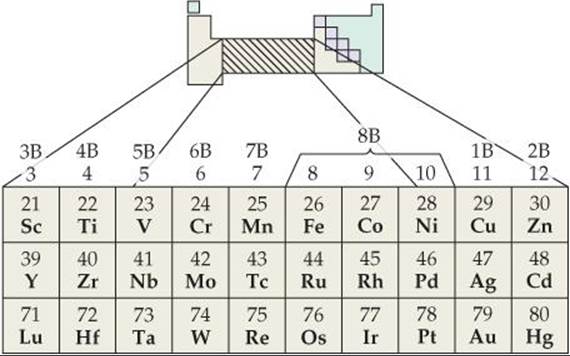
![]() FIGURE 23.1 The position of the transition metals in the periodic table. They are the B groups in periods 4, 5, and 6.
FIGURE 23.1 The position of the transition metals in the periodic table. They are the B groups in periods 4, 5, and 6.
Most transition metals in minerals have oxidation states of +1, +2, or +3. To obtain a pure metal from its mineral, various chemical processes must be performed to reduce the metal to the 0 oxidation state. Metallurgy is the science and technology of extracting metals from their natural sources and preparing them for practical use. It usually involves several steps: (1) mining, that is, removing the relevant ore (a mixture of minerals) from the ground, (2) concentrating the ore or otherwise preparing it for further treatment, (3) reducing the ore to obtain the free metal, (4) purifying the metal, and (5) mixing it with other elements to modify its properties. This last process produces an alloy, a metallic material composed of two or more elements. ![]() (Section 12.3)
(Section 12.3)
Physical Properties
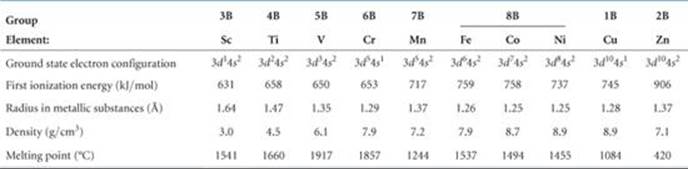
Some physical properties of the period 4 (also known as “first-row”) transition metals are listed in ![]() TABLE 23.2. The properties of the heavier transition metals vary similarly across periods 5 and 6.
TABLE 23.2. The properties of the heavier transition metals vary similarly across periods 5 and 6.
![]() FIGURE 23.2 shows the atomic radius observed in close-packed metallic structures as a function of group number.* The trends seen in the graph are a result of two competing forces. On the one hand, increasing effective nuclear charge favors a decrease in radius as we move left to right across each period.
FIGURE 23.2 shows the atomic radius observed in close-packed metallic structures as a function of group number.* The trends seen in the graph are a result of two competing forces. On the one hand, increasing effective nuclear charge favors a decrease in radius as we move left to right across each period. ![]() (Section 7.2) On the other hand, the metallic bonding strength increases until we reach the middle of each period and then decreases as we fill an-tibonding orbitals.
(Section 7.2) On the other hand, the metallic bonding strength increases until we reach the middle of each period and then decreases as we fill an-tibonding orbitals. ![]() (Section 12.4) As a general rule, a bond shortens as it becomes stronger.
(Section 12.4) As a general rule, a bond shortens as it becomes stronger. ![]() (Section 8.8) For groups 3B through 6B, these two effects work cooperatively and the result is a marked decrease in radius. In elements to the right of group 6B, the two effects counteract each other, reducing the decrease and eventually leading to an increase in radius.
(Section 8.8) For groups 3B through 6B, these two effects work cooperatively and the result is a marked decrease in radius. In elements to the right of group 6B, the two effects counteract each other, reducing the decrease and eventually leading to an increase in radius.
TABLE 23.1 • Principal Mineral Sources of Some Transition Metals
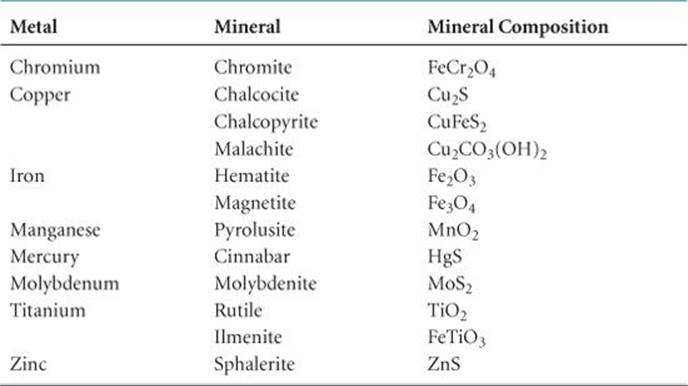
TABLE 23.2 • Properties of the Period 4 Transition Metals
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
Which element has the largest bonding atomic radius: Sc, Fe, or Au?
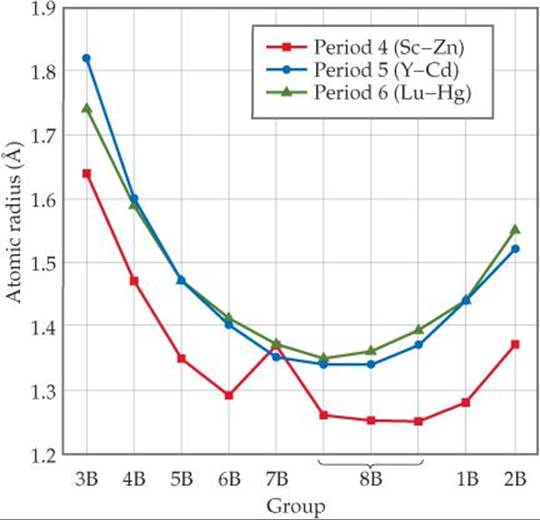
![]() FIGURE 23.2 Radii of transition metals as a function of group number.
FIGURE 23.2 Radii of transition metals as a function of group number.
In general, atomic radii increase as we move down in a family in the periodic table because of the increasing principal quantum number of the outer-shell electrons. ![]() (Section 7.3) Note in Figure 23.2, however, that once we move beyond the group 3B elements, the period 5 and period 6 transition elements in a given family have virtually the same radii. In group 5B, for example, tantalum in period 6 has virtually the same radius as niobium in period 5. This interesting and important effect has its origin in the lanthanide series, elements 57 through 70. The filling of 4forbitals through the lanthanide elements
(Section 7.3) Note in Figure 23.2, however, that once we move beyond the group 3B elements, the period 5 and period 6 transition elements in a given family have virtually the same radii. In group 5B, for example, tantalum in period 6 has virtually the same radius as niobium in period 5. This interesting and important effect has its origin in the lanthanide series, elements 57 through 70. The filling of 4forbitals through the lanthanide elements ![]() (Figure 6.31) causes a steady increase in the effective nuclear charge, producing a size decrease, called the lanthanide contraction, that just offsets the increase we expect as we go from period 5 transition metals to period 6. Thus, the period 5 and period 6 transition metals in each group have about the same radii all the way across the periodic table. Consequently, the period 5 and period 6 transition metals in a given group have similar chemical properties. For example, the chemical properties of the group 4B metals zirconium (period 5) and hafnium (period 6) are remarkably similar. These two metals always occur together in nature, and they are very difficult to separate.
(Figure 6.31) causes a steady increase in the effective nuclear charge, producing a size decrease, called the lanthanide contraction, that just offsets the increase we expect as we go from period 5 transition metals to period 6. Thus, the period 5 and period 6 transition metals in each group have about the same radii all the way across the periodic table. Consequently, the period 5 and period 6 transition metals in a given group have similar chemical properties. For example, the chemical properties of the group 4B metals zirconium (period 5) and hafnium (period 6) are remarkably similar. These two metals always occur together in nature, and they are very difficult to separate.
Electron Configurations and Oxidation States
Transition metals owe their location in the periodic table to the filling of the d subshells, as you saw in Figure 6.31. Many of the chemical and physical properties of transition metals result from the unique characteristics of the d orbitals. For a given transitionmetal atom, the valence (n - 1)dorbitals are smaller than the corresponding valence ns and np orbitals. In quantum mechanical terms, the (n - 1)d orbital wave functions drop off more rapidly as we move away from the nucleus than do the ns and np orbital wave functions. This characteristic feature of the d orbitals limits their interaction with orbitals on neighboring atoms, but not so much that they are insensitive to surrounding atoms. As a result, electrons in these orbitals behave sometimes like valence electrons and sometimes like core electrons. The details depend on location in the periodic table and the atom's environment.
![]() GO FIGURE
GO FIGURE
In which transition-metal ion of this group are the 3d orbitals completely filled?

![]() FIGURE 23.3 Aqueous solutions of transition metal ions. Left to right: Co2+, Ni2+, Cu2+, and Zn2+. The counterion is nitrate in all cases.
FIGURE 23.3 Aqueous solutions of transition metal ions. Left to right: Co2+, Ni2+, Cu2+, and Zn2+. The counterion is nitrate in all cases.
When transition metals are oxidized, they lose their outer s electrons before they lose electrons from the d subshell. ![]() (Section 7.4) The electron configuration of Fe is [Ar]3d6 4s2, for example, whereas that of Fe2+ is [Ar]3d5. Formation of Fe3+ requires loss of one 3d electron, giving [Ar]3d5. Most transition-metal ions contain partially occupied d subshells, which are responsible in large part for three characteristics:
(Section 7.4) The electron configuration of Fe is [Ar]3d6 4s2, for example, whereas that of Fe2+ is [Ar]3d5. Formation of Fe3+ requires loss of one 3d electron, giving [Ar]3d5. Most transition-metal ions contain partially occupied d subshells, which are responsible in large part for three characteristics:
1. Transition metals often have more than one stable oxidation state.
2. Many transition-metal compounds are colored, as shown in ![]() FIGURE 23.3.
FIGURE 23.3.
3. Transition metals and their compounds often exhibit magnetic properties.
![]() GO FIGURE
GO FIGURE
Why does the maximum oxidation state increase linearly from Sc through Mn?
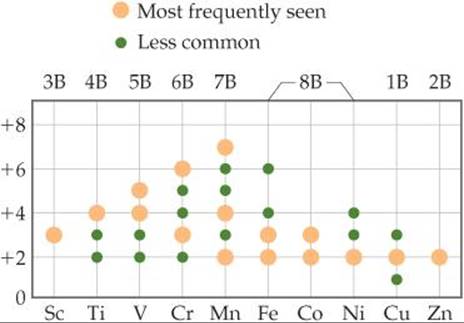
![]() FIGURE 23.4 Nonzero oxidation states of the period 4 transition metals.
FIGURE 23.4 Nonzero oxidation states of the period 4 transition metals.
![]() FIGURE 23.4 shows the common nonzero oxidation states for the period 4 transition metals. The +2 oxidation state, which is common for most transition metals, is due to the loss of the two outer 4s electrons. This oxidation state is found for all these elements except Sc, where the 3+ ion with an [Ar] configuration is particularly stable.
FIGURE 23.4 shows the common nonzero oxidation states for the period 4 transition metals. The +2 oxidation state, which is common for most transition metals, is due to the loss of the two outer 4s electrons. This oxidation state is found for all these elements except Sc, where the 3+ ion with an [Ar] configuration is particularly stable.
Oxidation states above +2 are due to successive losses of 3d electrons. From Sc through Mn the maximum oxidation state increases from +3 to +7, equaling in each case the total number of 4s plus 3d electrons in the atom. Thus, manganese has a maximum oxidation state of 2 + 5 = +7. As we move to the right beyond Mn in Figure 23.4, the maximum oxidation state decreases. This decrease is due in part to the attraction of d orbital electrons to the nucleus, which increases faster than the attraction of the s orbital electrons to the nucleus as we move left to right across the periodic table. In other words, in each period the d electrons become more corelike as the atomic number increases. By the time we get to zinc, it is not possible to remove electrons from the 3d orbitals through chemical oxidation.
In the transition metals of periods 5 and 6, the increased size of the 4d and 5d orbitals makes it possible to attain maximum oxidation states as high as +8, which is achieved in RuO4 and OsO4. In general, the maximum oxidation states are found only when the metals are combined with the most electronegative elements, especially O, F, and in some cases Cl.
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
Why doesn't Ti5+ exist?
Magnetism
The spin an electron possesses gives the electron a magnetic moment, a property that causes the electron to behave like a tiny magnet. In a diamagnetic solid, defined as one in which all the electrons in the solid are paired, the spin-up and spin-down electrons cancel one another. ![]() (Section 9.8) Diamagnetic substances are generally described as being nonmagnetic, but when a diamagnetic substance is placed in a magnetic field, the motions of the electrons cause the substance to be very weakly repelled by the magnet. In other words, these supposedly nonmagnetic substances do show some very faint magnetic character in the presence of a magnetic field.
(Section 9.8) Diamagnetic substances are generally described as being nonmagnetic, but when a diamagnetic substance is placed in a magnetic field, the motions of the electrons cause the substance to be very weakly repelled by the magnet. In other words, these supposedly nonmagnetic substances do show some very faint magnetic character in the presence of a magnetic field.
A substance in which the atoms or ions have one or more unpaired electrons is paramagnetic. ![]() (Section 9.8) In a paramagnetic solid, the electrons on one atom or ion do not influence the unpaired electrons on neighboring atoms or ions. As a result, the magnetic moments on the atoms or ions are randomly oriented, as shown in
(Section 9.8) In a paramagnetic solid, the electrons on one atom or ion do not influence the unpaired electrons on neighboring atoms or ions. As a result, the magnetic moments on the atoms or ions are randomly oriented, as shown in ![]() FIGURE 23.5 (a). When a paramagnetic substance is placed in a magnetic field, however, the magnetic moments tend to align parallel to one another, producing a net attractive interaction with the magnet. Thus, unlike a diamagnetic substance, which is weakly repulsed by a magnetic field, a paramagnetic substance is attracted to a magnetic field.
FIGURE 23.5 (a). When a paramagnetic substance is placed in a magnetic field, however, the magnetic moments tend to align parallel to one another, producing a net attractive interaction with the magnet. Thus, unlike a diamagnetic substance, which is weakly repulsed by a magnetic field, a paramagnetic substance is attracted to a magnetic field.
![]() GO FIGURE
GO FIGURE
Describe how the representation shown for the paramagnetic material would have to be changed if the material were placed in a magnetic field.
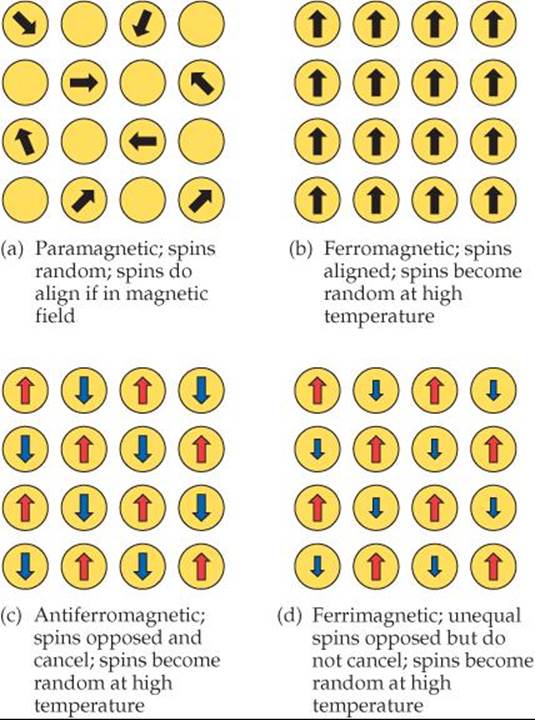
![]() FIGURE 23.5 The relative orientation of electron spins in various types of magnetic substances.
FIGURE 23.5 The relative orientation of electron spins in various types of magnetic substances.
When you think of a magnet, you probably picture a simple iron magnet. Iron exhibits ferromagnetism, a form of magnetism much stronger than paramagnetism. Ferromagnetism arises when the unpaired electrons of the atoms or ions in a solid are influenced by the orientations of the electrons in neighboring atoms or ions. The most stable (lowest-energy) arrangement is when the spins of electrons on adjacent atoms or ions are aligned in the same direction, as in Figure 23.5(b). When a ferromagnetic solid is placed in a magnetic field, the electrons tend to align strongly in a direction parallel to the magnetic field. The attraction to the magnetic field that results may be as much as one million times stronger than that for a paramagnetic substance.
When a ferromagnet is removed from an external magnetic field, the interactions between the electrons cause the ferromagnetic substance to maintain a magnetic moment. We then refer to it as a permanent magnet (![]() FIGURE 23.6).
FIGURE 23.6).
The only ferromagnetic transition metals are Fe, Co, and Ni, but many alloys also exhibit ferromagnetism, which is in some cases stronger than the ferromagnetism of the pure metals. Particularly powerful ferromagnetism is found in compounds containing both transition metals and lanthanide metals. Two of the most important examples are SmCo5 and Nd2Fe14B.

![]() FIGURE 23.6 A permanent magnet. Permanent magnets are made from ferromagnetic and ferrimagnetic materials.
FIGURE 23.6 A permanent magnet. Permanent magnets are made from ferromagnetic and ferrimagnetic materials.
Two additional types of magnetism involving ordered arrangements of unpaired electrons are depicted in Figure 23.5. In materials that exhibit antiferromagnetism [Figure 23.5(c)], the unpaired electrons on a given atom or ion align so that their spins are oriented in the direction opposite the spin direction on neighboring atoms. This means that the spin-up and spin-down electrons cancel each other. Examples of antiferromagnetic substances are chromium metal, FeMn alloys, and such transitionmetal oxides as Fe2O3, LaFeO3, and MnO.
A substance that exhibits ferrimagnetism [Figure 23.5(d)] has both ferromagnetic and antiferromagnetic properties. Like an antiferromagnet, the unpaired electrons align so that the spins in adjacent atoms or ions point in opposite directions. However, unlike an antiferromagnet, the net magnetic moments of the spin-up electrons are not fully canceled by the spin-down electrons. This can happen because the magnetic centers have different numbers of unpaired electrons (NiMnO3), because the number of magnetic sites aligned in one direction is larger than the number aligned in the other direction (Y3Fe5O12), or because both these conditions apply (Fe3O4). Because the magnetic moments do not cancel, the properties of ferrimagnetic materials are similar to the properties of ferromagnetic materials.
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
How do you think spin-spin interactions of unpaired electrons on adjacent atoms in a substance are affected by the interatomic distance?
Ferromagnets, ferrimagnets, and antiferromagnets all become paramagnetic when heated above a critical temperature. This happens when the thermal energy is sufficient to overcome the forces determining the spin directions of the electrons. This temperature is called the Curie temperature, TC, for ferromagnets and ferrimagnets and the Néel temperature, TN, for antiferromagnets.