CHEMISTRY THE CENTRAL SCIENCE
24 THE CHEMISTRY OF LIFE: ORGANIC AND BIOLOGICAL CHEMISTRY
CHAPTER SUMMARY AND KEY TERMS
INTRODUCTION AND SECTION 24.1 This chapter introduces organic chemistry, which is the study of carbon compounds (typically compounds containing carbon–carbon bonds), and biochemistry, which is the study of the chemistry of living organisms. We have encountered many aspects of organic chemistry in earlier chapters. Carbon forms four bonds in its stable compounds. The C—C single bonds and the C—H bonds tend to have low reactivity. Those bonds that have a high electron density (such as multiple bonds or bonds with an atom of high electronegativity) tend to be the sites of reactivity in an organic compound. These sites of reactivity are called functional groups.
SECTION 24.2 The simplest types of organic compounds are hydrocarbons, those composed of only carbon and hydrogen. There are four major kinds of hydrocarbons: alkanes, alkenes, alkynes, and aromatic hydrocarbons. Alkanes are composed of only C—H and C—C single bonds.Alkenes contain one or more carbon–carbon double bonds. Alkynes contain one or more carbon–carbon triple bonds. Aromatic hydrocarbons contain cyclic arrangements of carbon atoms bonded through both σ and delocalized π bonds. Alkanes are saturated hydrocarbons; the others are unsaturated.
Alkanes may form straight-chain, branched-chain, and cyclic arrangements. Isomers are substances that possess the same molecular formula but differ in the arrangements of atoms. In structural isomers the bonding arrangements of the atoms differ. Different isomers are given different systematic names. The naming of hydrocarbons is based on the longest continuous chain of carbon atoms in the structure. The locations of alkyl groups, which branch off the chain, are specified by numbering along the carbon chain.
Alkanes with ring structures are called cycloalkanes. Alkanes are relatively unreactive. They do, however, undergo combustion in air, and their chief use is as sources of heat energy produced by combustion.
SECTION 24.3 The names of alkenes and alkynes are based on the longest continuous chain of carbon atoms that contains the multiple bond, and the location of the multiple bond is specified by a numerical prefix. Alkenes exhibit not only structural isomerism but geometric (cis-trans) isomerism as well. In geometric isomers the bonds are the same, but the molecules have different geometries. Geometric isomerism is possible in alkenes because rotation about the C ═ C double bond is restricted.
Alkenes and alkynes readily undergo addition reactions to the carbon–carbon multiple bonds. Additions of acids, such as HBr, proceed via a rate-determining step in which a proton is transferred to one of the alkene or alkyne carbon atoms. Addition reactions are difficult to carry out with aromatic hydrocarbons, but substitution reactions are easily accomplished in the presence of catalysts.
SECTION 24.4 The chemistry of organic compounds is dominated by the nature of their functional groups. The functional groups we have considered are R, R', and R” represent hydrocarbon groups—for example, methyl (CH3) or phenyl (C6H5).
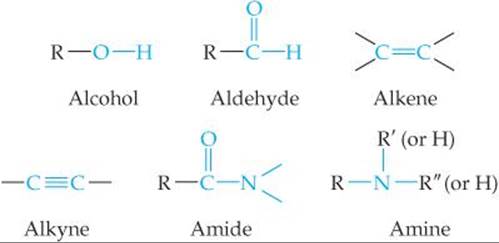
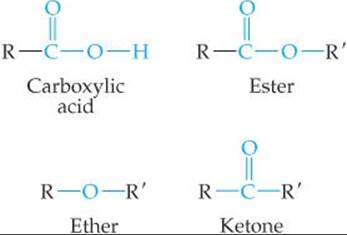
Alcohols are hydrocarbon derivatives containing one or more OH groups. Ethers are formed by a condensation reaction of two molecules of alcohol. Several functional groups contain the carbonyl (C═O) group, including aldehydes, ketones, carboxylic acids, esters, and amides. Aldehydes and ketones can be produced by oxidation of certain alcohols. Further oxidation of the aldehydes produces carboxylic acids. Carboxylic acids can form esters by a condensation reaction with alcohols, or they can form amides by a condensation reaction with amines. Esters undergohydrolysis (saponification) in the presence of strong bases.
SECTION 24.5 Molecules that possess nonsuperimposable mirror images are termed chiral. The two nonsuperimposable forms of a chiral molecule are called enantiomers. In carbon compounds a chiral center is created when all four groups bonded to a central carbon atom are different, as in 2-bromobutane. Many of the molecules occurring in living systems, such as the amino acids, are chiral and exist in nature in only one enantiomeric form. Many drugs of importance in human medicine are chiral, and the enantiomers may produce very different biochemical effects. For this reason, synthesis of only the effective isomers of chiral drugs has become a high priority.
SECTIONS 24.6 AND 24.7 Many of the molecules that are essential for life are large natural polymers that are constructed from smaller molecules called monomers. Three of these biopolymers are considered in this chapter: proteins, polysaccharides (carbohydrates), and nucleic acids.
Proteins are polymers of amino acids. They are the major structural materials in animal systems. All naturally occurring proteins are formed from 22 amino acids, although only 20 are common. The amino acids are linked by peptide bonds. A polypeptide is a polymer formed by linking many amino acids by peptide bonds.
Amino acids are chiral substances. Usually only one of the enantiomers is found to be biologically active. Protein structure is determined by the sequence of amino acids in the chain (its primary structure), the coiling or stretching of the chain (its secondary structure), and the overall shape of the complete molecule (its tertiary structure). Two important secondary structures are the α-helix and the β sheet. The process by which a protein assumes its biologically active tertiary structure is called folding. Sometimes several proteins aggregate together to form aquaternary structure.
SECTIONS 24.8 AND 24.9 Carbohydrates, which are polyhydroxy aldehydes and ketones, are the major structural constituents of plants and are a source of energy in both plants and animals. Glucose is the most common monosaccharide, or simple sugar. Two monosaccha-rides can be linked together by means of a condensation reaction to form a disaccharide. Polysaccharides are complex carbohydrates made up of many monosaccharide units joined together. The three most important polysaccharides are starch, which is found in plants; glycogen, which is found in mammals; and cellulose, which is also found in plants.
Lipids are compounds derived from glycerol and fatty acids and include fats and phospholipids. Fatty acids can be saturated, unsaturated, cis, or trans depending on their chemical formulas and structures.
SECTION 24.10 Nucleic acids are biopolymers that carry the genetic information necessary for cell reproduction; they also control cell development through control of protein synthesis. The building blocks of these biopolymers are nucleotides. There are two types of nucleic acids, theribonucleic acids (RNA) and the deoxyribonucleic acids (DNA). These substances consist of a polymeric backbone of alternating phosphate and ribose or deoxyribose sugar groups with organic bases attached to the sugar molecules. The DNA polymer is a double-stranded helix (double helix) held together by hydrogen bonding between matching organic bases situated across from one another on the two strands. The hydrogen bonding between specific base pairs is the key to gene replication and protein synthesis.
KEY SKILLS
• Distinguish among alkanes, alkenes, alkynes, and aromatic hydrocarbons. (Section 24.2)
• Draw hydrocarbon structures based on their names and name hydrocarbons based on their structures. (Sections 24.2 and 24.3)
• Distinguish between addition reactions and substitution reactions. (Section 24.3)
• Know the structures of the functional groups: alkene, alkyne, alcohol, carbonyl, ether, aldehyde, ketone, carboxylic acid, amine, amide. (Section 24.4)
• Understand what makes a compound chiral and be able to recognize a chiral substance. (Section 24.5)
• Recognize the amino acids and understand how they form peptides and proteins via amide bond formation. (Section 24.7)
• Understand the differences among the primary, secondary, tertiary, and quaternary structures of proteins. (Section 24.7)
• Be able to explain the difference between α-helix and β-sheet peptide and protein structures. (Section 24.7)
• Understand the distinction between starch and cellulose structures. (Section 24.8)
• Classify molecules as saccharides or lipids based on their structures. (Sections 24.8 and 24.9)
• Understand the difference between a saturated and unsaturated fat. (Section 24.9)
• Understand the structure of nucleic acids and the role played by complementary bases in DNA replication. (Section 24.10)