CHEMISTRY THE CENTRAL SCIENCE
ANSWERS TO GIVE IT SOME THOUGHT
CHAPTER 1
page 5 (a) 100 (b) atoms
page 10 Water is composed of two types of atoms: hydrogen and oxygen. Hydrogen is composed only of hydrogen atoms, and oxygen is composed only of oxygen atoms. Therefore, hydrogen and oxygen are elements and water is a compound.
page 13 (a) Chemical change: Carbon dioxide and water are different compounds than sugar. (b) Physical change: Water in the gas phase becomes water in the solid phase (frost). (c) Physical change: Gold in the solid state becomes liquid and then resolidifies.
page 16 pg, picogram (10–12 g)
page 19 2.5 × 102 m3 is, because it has units of length to the third power.
page 21 (b) Mass of a penny
page 27 Use all digits given in the conversion factor. Conversion factors may be exact and then have “infinite” significant digits (for example, 2.54cm = 1 inch exactly). Usually, your answer will have its number of significant digits limited by those of the quantities given in the problem.
CHAPTER 2
page 41 (a) The law of multiple proportions. (b) The second compound must contain two oxygen atoms for each carbon atom (that is, twice as many carbon atoms as the first compound).
page 44 Most α particles pass through the foil without being deflected because most of the volume of the atoms that comprise the foil is empty space.
page 45 (a) The atom has 15 electrons because atoms have equal numbers of electrons and protons. (b) The protons reside in the nucleus of the atom.
page 48 Any single atom of chromium must be one of the isotopes of that element. The isotope mentioned has a mass of 52.94 amu and is probably 53Cr. The atomic weight differs from the mass of any particular atom because it is the average atomic mass of the naturally occurring isotopes of the element.
page 51 (a) Cl, (b) third period and group 7A, (c) 17, (d) nonmetal
page 54 (a) C2H6, (b) CH3, (c) Probably the ball-and-stick model because the angles between the sticks indicate the angles between the atoms
page 57 We write the empirical formulas for ionic compounds. Thus, the formula is CaO.
page 60 (a) The transition metals can form more than one type of cation, and the charges of these ions are therefore indicated explicitly with Roman numerals: Chromium(II) ion is Cr2+. Calcium, on the other hand, always forms the Ca2+ ion, so there is no need to distinguish it from other calcium ions with different charges. (b) The –ium ending indicates that the ion is formed from nonmetals.
page 61 An -ide ending usually means a monatomic anion, although there are some anions with two atoms that are also named this way. An -ate ending indicates an oxyanion. The most common oxyanions have the -ate ending. An -ite ending also indicates an oxyanion, but one having less O than the anion whose name ends in -ate.
page 62 BO33– and SiO44–. The borate has three O atoms, like the other oxyanions of the second period in Figure 2.27, and its charge is 3–, following the trend of increasing negative charge as you move to the left in the period. The silicate has four O atoms, as do the other oxyanions in the third period in Figure 2.25, and its charge is 4–, also following the trend of increasing charge moving to the left.
page 65 Iodic acid, by analogy to the relationship between the chlorate ion and chloric acid
page 67
CHAPTER 3
page 78 Each Mg(OH)2 has 1 Mg, 2 O, and 2 H; thus, 3 Mg(OH)2 represents 3 Mg, 6 O, and 6 H.
page 83 The product is an ionic compound involving Na+ and S2–, and its chemical formula is therefore Na2S.
page 88 (a) A mole of glucose. By inspecting their chemical formulas we find that glucose has more atoms of H and O than water and in addition it also has C atoms. Thus, a molecule of glucose has a greater mass than a molecule of water. (b) They both contain the same number of molecules because a mole of each substance contains 6.02 × 1023 molecules.
page 93 The N:H ratio is 2:4 = 1:2.
page 96 There are experimental uncertainties in the measurements.
page 97 3.14 mol because 2 mol H2![]() 1 mol O2 based on the coefficients in the balanced equation
1 mol O2 based on the coefficients in the balanced equation
page 98 The number of grams of product formed is the sum of the masses of the two reactants, 50 g. When two substances react in a combination reaction, only one substance is formed as a product. According to the law of conservation of mass, the mass of the product must equal the masses of the two reactants.
CHAPTER 4
page 118 (a) K+(aq) and CN–(aq), (b) Na+(aq) and ClO4–(aq)
page 119 NaOH because it is the only solute that is a strong electrolyte
page 123 Na+(aq) and NO3–(aq)
page 125 Three. Each COOH group will partially ionize in water to form H+(aq).
page 126 Only soluble metal hydroxides are classified as strong bases and Al(OH)3 is insoluble.
page 130 SO2(g)
page 133 (a) –3, (b) +5
page 136 (a) Yes, nickel is below zinc in the activity series so Ni2+ (aq) will oxidize Zn(s) to form Ni(s) and Zn2+ (aq). (b) No reaction will occur because the Zn2+ (aq) ions cannot be further oxidized.
page 139 The second solution is more concentrated, 2.50 M, than the first solution, which has a concentration of 1.00 M.
page 142 The concentration is halved to 0.25 M.
CHAPTER 5
page 162 No. The potential energy is lower at the bottom of the hill. (b) Once the bike comes to a stop, its kinetic energy is zero, just as it was at the top of the hill.
page 163 Open system. Humans exchange matter and energy with their surroundings.
page 167 Endothermic
page 169 The balance (current state) does not depend on the ways the money may have been transferred into the account or on the particular expenditures made in withdrawing money from the account. It depends only on the net total of all the transactions.
page 169 Because E, P, and V are state functions that don't depend on path, H = E + PV must also be a state function.
page 170 No. If Δ V is zero, then the expression w = –PΔV is also zero.
page 171 A thermometer to measure temperature changes
page 173 No. Because only half as much matter is involved, the value of ΔH would be ![]() (–483.6 kJ) = –241.8 kJ.
(–483.6 kJ) = –241.8 kJ.
page 176 Hg(l). Rearranging Equation 5.22 gives ![]() . When q and m are constant for a series of substances, then
. When q and m are constant for a series of substances, then ![]() . Therefore, the element with the smallest Cs in Table 5.2 has the largest ΔT, Hg(l).
. Therefore, the element with the smallest Cs in Table 5.2 has the largest ΔT, Hg(l).
page 181 (a) The sign of ΔH changes. (b) The magnitude of ΔH doubles.
page 184 No. Because O3(g) is not the most stable form of oxygen at 25 °C, 1 atm [O2(g) is], ![]() for O3(g) is not necessarily zero. In Appendix C we see that it is 142.3 kJ/mol.
for O3(g) is not necessarily zero. In Appendix C we see that it is 142.3 kJ/mol.
page 189 Fats, because they have the largest fuel value of the three
page 191 Combustion of H2(g) produces only H2O(g). No CO2(g) or other gases that might contribute to climate change issues are produced.
CHAPTER 6
page 210 No. Both visible light and X-rays are forms of electromagnetic radiation. They therefore both travel at the speed of light, c. Their differing ability to penetrate skin is due to their different energies, which we will discuss in the next section.
page 211 E =hv = (6.63 × 10–34 J-s)(5 × 10–30 s–1) = 3 × 10–30 J; this radiation cannot produce a burst of 5 × 10–36J because it can only produce energy in multiples of 3 × 10–30 J.
page 212 Ultraviolet. Figure 6.4 shows that a photon in the ultraviolet region of the electromagnetic spectrum has a higher frequency and therefore a greater energy than a photon in the infrared region.
page 214 According to the third postulate, photons of only certain allowed frequencies can be absorbed or emitted as the electron changes energy state. The lines in the spectrum correspond to the allowed frequencies.
page 215 Absorb, because it is moving from a lower-energy state (n = 3) to a higher-energy state (n = 7)
page 217 Yes, all moving objects produce matter waves, but the wavelengths associated with macroscopic objects, such as the baseball, are too small to allow for any way of observing them.
page 219 The small size and mass of subatomic particles. The term h/4π in the uncertainty principle is a very small number that becomes important only when considering extremely small objects, such as electrons.
page 220 Bohr proposed that the electron in the hydrogen atom moves in a well-defined circular path around the nucleus (an orbit). In the quantum-mechanical model, no effort is made to describe the motion of the electron. An orbital is a wave function related to the probability of finding the electron at any point in space.
page 221 The energy of an electron in the hydrogen atom is proportional to –1/n2, as seen in Equation 6.5. The difference between –1/(2)2 and –1/(1)2 is much greater than the difference between –1/(3)2 and –1(2)2.
page 226 (a) There is one 3s orbital, three 3p orbitals, and ten 3d orbitals, for a total of 14 orbitals. (b) 3s < 3p < 3d.
page 232 The 6s orbital, which starts to hold electrons at element 55, Cs
page 237 We can't conclude anything! Each of the three elements has a different valence electron configuration for its (n – 1)d and ns subshells: For Ni, 3d84s2; for Pd, 4d10; and for Pt, 5d96s1.
CHAPTER 7
page 251 Atomic number is governed by the number of protons in the nucleus, but atomic weight is governed by both the number of protons and neutrons in the nucleus (electrons are too light to worry about). Co/Ni, Cu/Zn, and Te/I are other pairs of elements whose atomic weights are “off” compared to their atomic numbers.
page 254 The 2p electron in a Ne atom would experience a larger Zeff than the 3s electron in Na, due to the better shielding by all the 2s and 2p electrons for Na's 3s electron.
page 256 These trends work against each other: Zeff increasing would imply that the valence electrons are pulled tighter in to make the atom smaller, while orbital size “increasing” would imply that atomic size would also increase. The orbital size effect is larger: As you go down a column in the periodic table, atomic size generally increases.
page 259 It is harder to remove another electron from Na+, so the process in Equation 7.3 would require more energy and, hence, shorter-wavelength light (see Sections 6.1 and 6.2).
page 260 Since Zeff increases as you go from boron to carbon, we would expect that the first ionization energy would be larger for carbon. Therefore, I2 for C is even greater.
page 262 The same
page 264 The numbers are the same; the signs are opposite.
page 265 Increasing metallic character is correlated with decreasing ionization energy.
page 268 Since the melting point is so low, we would expect a molecular rather than ionic compound. Thus, so PCl3 is more likely than ScCl3.
page 270 Its low ionization energy
page 272 In the acidic environment of the stomach, carbonate can react to give carbonic acid, which decomposes to water and carbon dioxide gas.
page 274 The longest wavelength of visible light is about 750 nm (Section 6.1). We can assume that this corresponds to the lowest energy of light (since E = hc/λ) needed to break bonds in hydrogen peroxide. If we plug in 750 nm for λ, we can calculate the energy to break one OO bond in one molecule of hydrogen peroxide, in joules. If we multiple by Avogadro's number, we can calculate how many joules it would take to break a mole of OO bonds in hydrogen peroxide (which is the number one normally finds).
page 275 The halogens all have ground-state electron configurations that are ns2np5; sharing an electron with only one other atom makes stable compounds.
page 276 We can estimate the radius to be 1.5 Å, and the first ionization energy to be 900 kJ/mol. In fact, its bonding radius is indeed 1.5 Å, and the experimental ionization energy is 920 kJ/mol.
CHAPTER 8
page 290 No. Cl has seven valence electrons. The first and second Lewis symbols are both correct—they both show seven valence electrons, and it doesn't matter which of the four sides has the single electron. The third symbol shows only five electrons and is incorrect.
page 292 CaF2 is an ionic compound consisting of Ca2+ and F– ions. When Ca and F2 react to form CaF2, each Ca atom loses two electrons to form a Ca2+ ion and each fluorine atom in F2 takes up an electron, forming two F– ions. Thus, we can say that each Ca atom transfers one electron to each of two fluorine atoms.
page 292 No. Figure 7.9 shows that the alkali metal with the smallest first ionization energy is Cs with a value of +376 kJ/mol. Figure 7.11 shows that the halogen with the largest electron affinity is Cl with a value of –349 kJ/mol. The sum of the two energies gives a positive energy (endothermic). Therefore, all other combinations of alkali metals with halogens will also have positive values.
page 296 Rhodium, Rh
page 297 Weaker. In both H2 and H2+ the two H atoms are principally held together by the electrostatic attractions between the nuclei and the electron(s) concentrated between them. H2+ has only one electron between the nuclei whereas H2 has two and this results in the H — H bond in H2 being stronger.
page 298 Triple bond. CO2 has two C — O double bonds. Because the C — O bond in carbon monoxide is shorter, it is likely to be a triple bond.
page 299 Electron affinity measures the energy released when an isolated atom gains an electron to form a 1 – ion. The electronegativity measures the ability of the atom to hold on to its own electrons and attract electrons from other atoms in compounds.
page 300 Polar covalent. The difference in electronegativity between S and O is 3.5 – 2.5 = 1.0. Based on the examples of F2, HF, and LiF, the difference in electronegativity is great enough to introduce some polarity to the bond but not sufficient to cause a complete electron transfer from one atom to the other.
page 302 IF. Because the difference in electronegativity between I and F is greater than that between Cl and F, the magnitude of Q should be greater for IF. In addition, because I has a larger atomic radius than Cl, the bond length in IF is longer than that in ClF. Thus, both Q and r are larger for IF and, therefore, μ = Qr will be larger for IF.
page 303 Smaller dipole moment for C — H. The magnitude of Q should be similar for C — H and H — I bonds because the difference in electronegativity for each bond is 0.4. The C — H bond length is 1.1 Å and the H — I bond length is 1.6 Å. Therefore μ = Qr will be greater for H — I because it has a longer bond (larger r).
page 304 OsO4. The data suggest that the yellow substance is a molecular species with its low melting and boiling points. Os in OsO4 has an oxidation number of +8 and Cr in Cr2O3 has an oxidation number of +3. In Section 8.4, we learn that a compound with a metal in a high oxidation state should show a high degree of covalence and OsO4 fits this situation.
page 308 There is probably a better choice of Lewis structure than the one chosen. Because the formal charges must add up to 0 and the formal charge on the F atom is +1, there must be an atom that has a formal charge of –1. Because F is the most electronegative element, we don't expect it to carry a positive formal charge.
page 310 Yes. There are two resonance structures for ozone that each contribute equally to the overall description of the molecule. Each O — O bond is therefore an average of a single bond and a double bond, which is a “one-and-a-half” bond.
page 310 As “one-and-a-third” bonds. There are three resonance structures, and each of the three N — O bonds is single in two of those structures and double in the third. Each bond in the actual ion is an average of these: (1 + 1 + 2)/3 = 1![]() .
.
page 312 No, it will not have multiple resonance structures. We can't “move” the double bonds, as we did in benzene, because the positions of the hydrogen atoms dictate specific positions for the double bonds. We can't write any other reasonable Lewis structures for the molecule.
page 312 The formal charge of each atom is shown here:
![]()
The first structure shows each atom with a zero formal charge and therefore it is the dominant Lewis structure. The second one shows a positive formal charge for an oxygen atom, which is a highly electronegative atom, and this is not a favorable situation.
page 315 The atomization of ethane produces 2 C(g) + 6 H(g). In this process, six C — H bonds and one C — C bond are broken. We can use 6D(C — H) to estimate the amount of enthalpy needed to break the six C — H bonds. The difference between that number and the enthalpy of atomization is an estimate of the bond enthalpy of the C — C bond, D(C — C).
page 315 H2O2. From Table 8.4, the bond enthalpy of the O — O single bond in H2O2 (146 kJ/mol) is much lower than that of the O ═ O bond in O2 (495 kJ/mol). The weaker bond in H2O2 is expected to make it more reactive than O2.
CHAPTER 9
page 334 Octahedral. Removing two atoms that are opposite each other leads to a square-planar geometry.
page 335 The molecule does not follow the octet rule because it has ten electrons around the central A atom. There are four electron domains around A: two single bonds, one double bond, and one nonbonding pair.
page 336 Each of the three represents a single electron domain in the VSEPR model.
page 339 Yes. Based on one resonance structure, we might expect the electron domain that is due to the double bond to “push” the domains that are due to the single bonds, leading to angles slightly different from 120°. However, we must remember that there are two other equivalent resonance structures—each of the three O atoms has a double bond to N in one of the three resonance structures (Section 8.6). Because of resonance, all three O atoms are equivalent, and they will experience the same amount of repulsion, which leads to bond angles equal to 120°.
page 339 A tetrahedral arrangement of electron domains is preferred because the bond angles are 109.5° compared to 90°. bond angles in a square-planar arrangement of electron domains. The larger bond angles result in smaller repulsions among electron domains and a more stable structure.
page 343 Yes. The C — O and C — S bond dipoles exactly oppose each other, like in CO2, but because O and S have different electronegativities, the magnitudes of the bond dipoles will be different. As a consequence, the bond dipoles will not cancel each other and the OCS molecule has a nonzero dipole moment.
page 348 Both p orbitals are perpendicular to the Be —— F bond axes.
page 348 (bottom) The unhybridized p orbital is oriented perpendicular to the plane defined by the three sp2 hybrids (trigonal-planar array of lobes) with one lobe on each side of the plane.
page 353 The molecule should not be linear. Because there are three electron domains around each N atom, we expect sp2 hybridization and H — N — N angles of approximately 120°. The molecule is expected to be planar; the unhybridized 2p orbitals on the N atoms can form a π bond only if all four atoms lie in the same plane. You might notice that there are two ways in which the H atoms can be arranged: They can be both on the same side of the N ═ N bond or on opposite sides of the N ═ N bond.
page 358 The σ bond component is formed from sp hybrid orbitals.
page 360 The molecule would fall apart. With one electron in the bonding MO and one in the antibonding MO, there is no net stabilization of the electrons relative to two separate H atoms.
page 362 Yes. In Be2+ there would be two electrons in the σ2s MO but only one electron in the ![]() MO; therefore, the ion is predicted to have a bond order of
MO; therefore, the ion is predicted to have a bond order of ![]() . It should (and does) exist.
. It should (and does) exist.
page 366 No. If the σ2p MO were lower in energy than the π2p MOs, we would expect the σ2p MO to hold two electrons and the π2p MOs to hold one electron each, with the same spin. The molecule would therefore be paramagnetic.
CHAPTER 10
page 384 Small
page 385 1470 lb
page 389 It would be halved.
page 390 No—you have to convert T to Kelvin to calculate this properly.
page 392 Avogadro's number, 6.022 × 1023
page 396 Less dense
page 399 The pressure due to N2 would be the same, but the total pressure would increase.
page 404 HCl (slowest) < O2 < H2 (fastest)
page 406 3/2
page 409 (a) Decrease, (b) No change
page 410 (b) 100 K and 5 atm
page 411 They do have intermolecular attractions for each other, and they do take up space.
CHAPTER 11
page 430 CH4 < CCl4 < CBr4. Because all three molecules are nonpolar, the strength of dispersion forces determines the relative boiling points. Polarizability increases in order of increasing molecular size and molecular weight, CH4 < CCl4 < CBr4; hence, the dispersion forces and boiling points increase in the same order.
page 434 Ca(NO3)2 in water, because calcium nitrate is a strong electrolyte that forms ions and water is a polar molecule with a dipole moment. Ion–dipole forces cannot be present in a CH3OH/H2O mixture because CH3OH does not form ions.
page 438 (a) Both viscosity and surface tension decrease with increasing temperature because of the increased molecular motion. (b) Both properties increase as the strength of intermolecular forces increases.
page 440 Melting (or fusion), endothermic
page 443 CCl4. Both compounds are nonpolar; therefore, only dispersion forces exist between the molecules. Because dispersion forces are stronger for the larger, heavier CBr4, it has a lower vapor pressure than CCl4. The substance with the larger vapor pressure at a given temperature is more volatile.
CHAPTER 12
page 466 Tetragonal. There are two three–dimensional lattices that have a square base with a third vector perpendicular to the base, tetragonal and cubic, but in a cubic lattice the a, b, and c lattice vectors are all of the same length.
page 473 The packing efficiency decreases as the number of nearest neighbors decreases. The structures with the highest packing efficiency, hexagonal and cubic close packing, both have atoms with a coordination number of 12. Body–centered cubic packing, where the coordination number is 8, has a lower packing efficiency, and primitive cubic packing, where the coordination number is 6, has a lower packing efficiency still.
page 474 Interstitial, because boron is a small nonmetal atom that can fit in the voids between the larger palladium atoms
page 481 (a) Gold, Au. Tungsten, W, lies near the middle of the transition metal series where the bands arising from the d orbitals and the s orbital are approximately half–filled. This electron count should fill the bonding orbitals and leave the antibonding orbitals mostly empty. (b) Because both elements have similar numbers of electrons in the bonding orbitals but tungsten has fewer electrons in antibonding orbitals, it will have a higher melting point.
page 482 No. In a crystal the lattice points must be identical. Therefore, if an atom lies on top of a lattice point, then the same type of atom must lie on all lattice points. In an ionic compound there are at least two different types of atoms, and only one can lie on the lattice points.
page 484 Four. The empirical formula of potassium oxide is K2O. Rearranging Equation 12.1 we can determine the potassium coordination number to be anion coordination number × (number of anions per formula unit/number of cations per formula unit) = 8(1/2) = 4.
page 494 A condensation polymer. The presence of both —COOH and —NH2 groups allow molecules to react with one another forming C—N bonds and splitting out H2O.
page 495 As the vinyl acetate content increases more side chain branching occurs which inhibits the formation of crystalline regions thereby lowering the melting point.
page 498 No. The emitted photons have energies that are similar in energy to the band gap of the semiconductor. If the size of the crystals is reduced into the nanometer range, the band gap will increase. However, because 340–nm light falls in the UV region of the electromagnetic spectrum, increasing the energy of the band gap will only shift the light deeper into the UV.
CHAPTER 13
page 514 Energy (or enthalpy) and entropy
page 515 The lattice energy of NaCl(s) must be overcome to separate Na+ and Cl– ions and disperse them into a solvent. C6H14 is nonpolar. Interactions between ions and nonpolar molecules tend to be very weak. Thus, the energy required to separate the ions in NaCl is not recovered in the form of ion–C6H14 interactions.
page 517 (a) Separating solvent molecules from each other requires energy and is therefore endothermic. (b) Forming the solute– solvent interactions is exothermic.
page 519 The added solute provides a template for the solid to begin to crystallize from solution, and the excess dissolved solute comes out of solution leaving a saturated solution.
page 522 The solubility in water would be considerably lower because there would no longer be hydrogen bonding with water, which promotes solubility.
page 526 Dissolved gases become less soluble as temperature increases, and they come out of solution, forming bubbles below the boiling point of water.
page 526 230 ppm (1 ppm is 1 part in 106); 2.30 × 105 ppb (1 ppb is 1 part in 109).
page 528 For dilute aqueous solutions the molality will be nearly equal to the molarity. Molality is the number of moles of solute per kilogram of solvent, whereas molarity is the number moles of solute per liter of solution. Because the solution is dilute, the mass of solvent is essentially equal to the mass of the solution. Furthermore, a dilute aqueous solution will have a density of 1.0 kg/L. Thus, the number of liters of solution and the number of kilograms of solvent will be essentially equal.
page 531 The lowering of the vapor pressure depends on the total solute concentration (Equation 13.11). One mole of NaCl (a strong electrolyte) provides 2 mol of particles (1 mol of Na+ and 1 mol Cl), whereas one mole of (a nonelectrolyte) provides only 1 mol particles.
page 534 Not necessarily; if the solute is a strong or weak electrolyte, it could have a lower molality and still cause an increase of 0.51 °C. The total molality of all the particles in the solution is 1 m.
page 537 The 0.20-m solution is hypotonic with respect to the 0.5-m solution. (A hypotonic solution will have a lower concentration and hence a lower osmotic pressure.)
page 539 They would have the same osmotic pressure because they have the same concentration of particles. (Both are strong electrolytes that are 0.20 M in total ions.)
page 543 The smaller droplets carry negative charges because of the embedded stearate ions and thus repel one another.
CHAPTER 14
page 559 The rate will increase.
page 562 Average rate is for a large time interval; instantaneous rate is for an “instant” in time. Yes, they can have the same numeric value, especially if a plot of concentration versus time is linear.
page 565 (top) Reaction rate is what we measure as a reaction proceeds—change in concentration in time for one or more of the components in the mixture. Reaction rate always has units of concentration per time, usually M/s. A rate constant is what we calculate from reaction rate data, and its magnitude is proportional to the reaction rate, but its units depend on the reaction order. The rate law of a reaction is an equation that relates reaction rate to the rate constant: Rate = k[A]m[B]n, for components A and B in the reaction.
page 565 No. Rate is always change in concentration per time; rate constant has units that depend on the form of the rate law.
page 566 (a) The reaction is second order in NO, first order in H2, and third order overall. (b) No. Doubling NO concentration will quadruple the rate, but doubling H2 concentration will merely double the rate.
page 567 No reaction will take place.
page 573 1.25 g
page 575 The half-life will increase.
page 578 No—transition states are by definition not stable.
page 578 The collision may not have occurred with enough energy for reaction to occur, and/or the collision may not have occurred with the proper orientation of reactant molecules to favor product formation.
page 581 Bimolecular
page 585 Most reactions occur in elementary steps; the rate law is governed by the elementary steps, not by their sum (which is the overall balanced equation).
page 587 The odds of three molecules colliding with each other properly to react is very low.
page 590 By lowering the activation energy for the reaction or by increasing the frequency factor
page 591 A homogeneous catalyst will be harder to separate from the reaction mixture than a heterogeneous one.
page 593 People do say this, but we have to be careful. An enzyme-catalyzed reaction will have a lower transition state energy than the uncatalyzed reaction, but the nature of the transition state is probably different than the uncatalyzed version.
CHAPTER 15
page 614 (a) The rates of the forward and reverse reactions. (b) Greater than 1
page 614 When the concentrations of reactants and products are no longer changing
page 617 It does not depend on starting concentrations.
page 617 Units of moles/L are used to calculate Kc; units of partial pressure are used to calculate Kp.
page 618 0.00140
page 621 It is cubed.
page 623 Kp = PH2O
page 625 Kc = [NH4+][OH–]/[NH3]
page 633 (a) It shifts to the right. (b) It shifts to the left.
page 633 (bottom) It will shift to the left, the side with a larger number of moles of gas.
page 636 As the temperature increases, a larger fraction of molecules in the liquid phase have enough energy to overcome their intermolecular attractions and go into the vapor; the evaporation process is endothermic.
page 638 No
CHAPTER 16
page 652 The H+ ion for acids and the OH– ion for bases
page 654 CH3NH2 is the base because it accepts a H+ from H2S as the reaction moves from the left–hand to the right–hand side of the equation.
page 657 As the conjugate base of a strong acid, we would classify ClO4– as having negligible basicity.
page 661 pH is defined as –log[H+]. This quantity will become negative if the H+ concentration exceeds 1 M, which is possible. Such a solution would be highly acidic.
page 662 pH = 14.00 – 3.00 = 11.00. This solution is basic because pH > 7.0.
page 665 Both NaOH and Ba(OH)2 are soluble hydroxides. Therefore, the hydroxide concentrations will be 0.001 M for NaOH and 0.002 M for Ba(OH)2. Because the Ba(OH)2 solution has a higher [OH–], it is more basic and has a higher pH.
page 666 Because CH3– is the conjugate base of a substance that has negligible acidity, CH3– must be a strong base. Bases stronger than OH– abstract H+ from water molecules:
CH3– + H2O ![]() CH4 + OH–.
CH4 + OH–.
page 668 Oxygen
page 671 Because weak acids typically undergo very little ionization, often less than 1%. Normally we make this assumption and then check its validity based on the concentration of conjugate base formed in the calculation. If it is ≤5% of the initial concentration of the weak acid, we can generally use this assumption. If not, we must do an exact calculation.
page 674 This is the acid–dissociation constant for the loss of the third and final proton from H3PO4, corresponding to the equilibrium HPO42–![]() H+ + PO43–.
H+ + PO43–.
page 680 The pKa value is –log Ka = –log(6.8 × 10–4) = 3.17. The pKb value is 14.00 – p Ka = 14.00 – 3.17 = 10.83.
page 682 Nitrate is the conjugate base of nitric acid, HNO3. The con jugate base of a strong acid does not act as a base, so NO3– ions will not affect the pH. Carbonate is the conjugate base of hydrogen carbonate, HCO3–, which is a weak acid. The conjugate base of a weak acid acts as a weak base, so CO32– ions will increase the pH.
page 686 The increasing acidity going down a group is due mainly to decreasing H — X bond strength. The trend going across a period is due mainly to the increasing electronegativity of X, which weakens the H — X bond.
page 687 HBrO3. For an oxyacid, acidity increases as the electronegativity of the central ion increases, which would make HBrO2 more acidic than HIO2. Acidity also increases as the number of oxygens bound to the central atom increases, which would make HBrO3 more acidic than HBrO2. Combining these two relationships we can order these acids in terms of increasing acid-dissociation constant, HIO2 < HBrO2 < HBrO3.
page 689 The carboxyl group, — COOH
page 690 It must have an unshared pair of electrons that can be shared with another atom.
CHAPTER 17
page 707 (top) The Cl– ion is the only spectator ion. The pH is determined by the equilibrium NH3(aq) + H2O(l) ![]() OH–(aq) + NH4+(aq).
OH–(aq) + NH4+(aq).
page 707 (bottom) HNO3 and NO3–. To form a buffer we need comparable concentrations of a weak acid and its conjugate base. HNO3 and NO3– will not form a buffer because HNO3 is a strong acid and the NO3– ion is merely a spectator ion.
page 708 (a) The OH– of NaOH (a strong base) reacts with the acid member of the buffer (CH3COOH), abstracting a proton. Thus, [CH3COOH], decreases and [CH3COO–] increases. (b) The H+ of HCl (a strong acid) reacts with the base member of the buffer [CH3COOH–]. Thus, [CH3COO–] decreases and [CH3COOH] increases.
page 711 A buffer will be most resistant to changes in pH when the concentrations of the weak acid and its conjugate base are equal. When the two are exactly equal the Henderson–Hasselbach equation tells us that the pH of the buffer will be equal to the p Ka of the weak acid. p Ka The p Ka values of nitrous acid and hypochlorous acid are 3.35 and 7.52, respectively. Thus, HClO would be more suitable for a pH = 7.0 buffer solution. To make a buffer we would also need a salt containing ClO–, such as NaClO.
page 716 The pH = 7. The neutralization of a strong base with a strong acid gives a salt solution at the equivalence point. The salt contains ions that do not change the pH of water.
page 721 The following titration curve shows the titration of 25 mL of Na2CO3 with HCl, both with 0.1 M concentrations. The overall reaction between the two is
Na2CO3(aq) + HCl(aq) ![]() 2 NaCl(aq) + CO2(g) + H2O(l)
2 NaCl(aq) + CO2(g) + H2O(l)
The initial pH (sodium carbonate in water only) is near 11 because CO32– is a weak base in water. The graph shows two equivalence points, A and B. The first point, A, is reached at a pH of about 9:
Na2CO3(aq) + HCl(aq) ![]() NaCl(aq) + NaHCO3(aq)
NaCl(aq) + NaHCO3(aq)
HCO3– is weakly basic in water and is a weaker base than the carbonate ion. The second point, B, is reached at a pH of about 4:
NaHCO3(aq) + HCl(aq) ![]() NaCl(aq) + CO2(g) + H2O(l)
NaCl(aq) + CO2(g) + H2O(l)
H2CO3, a weak acid, forms and decomposes to carbon dioxide and water.
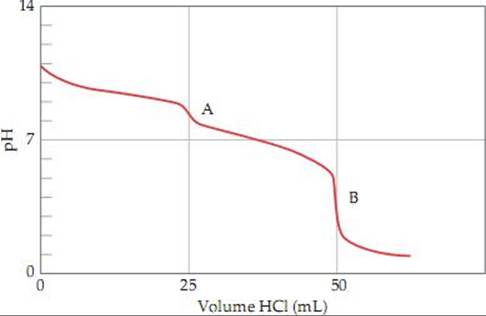
page 722 The nearly vertical portion of the titration curve at the equivalence point is smaller for a weak acid–strong base titration; as a result fewer indicators undergo their color change within this narrow range.
page 724 AgCl. Because all three compounds produce the same number of ions, their relative stabilities correspond directly to the Ksp values, with the compound with the largest Ksp value being the most soluble.
page 734 Amphoteric substances are insoluble in water but dissolve in the presence of sufficient acid or base. Amphiprotic substances can both donate and accept protons.
page 738 The solution must contain one or more of the cations in group 1 of the qualitative analysis scheme, Ag+, Pb2+ or Hg22+.
CHAPTER 18
page 754 Photoionization is a process in which a molecule breaks into ions upon illumination with light; photodissociation is a process in which molecules break up upon illumination with light but the products bear no charge.
page 755 Because those molecules do not absorb light at those wavelengths
page 757 Yes—Cl is neither a product nor a reactant in the overall reaction, and its presence does speed the reaction up.
page 760 SO2 in the atmosphere reacts with oxygen to form SO3. SO3 in the atmosphere reacts with water in the atmosphere to form H2SO4, sulfuric acid. The sulfuric acid dissolves in water droplets that fall to Earth, causing “acid rain” that has a pH of 4 or so.
page 761 NO2 photodissociates to NO and O; the O atoms react with O2 in the atmosphere to form ozone, which is a key ingredient in photochemical smog.
page 763 Higher humidity means there is more water in the air. Water absorbs infrared light, which we feel as heat. After sundown, the ground that has been warmed earlier in the day reradiates heat out. In locations with higher humidity, this energy is absorbed somewhat by the water and in turn is reradiated to some extent back to the Earth, resulting in warmer temperatures compared to a low-humidity location.
page 764 We need to be below water's critical point. Therefore, to sublime water we need to be below 0.006 atm. A wide range of temperatures will work for sublimation at this pressure—the most environmentally relevant ones are – 50 °C to 100 °C.
page 768 The pollutants are capable of being oxidized (either directly by reaction with dissolved oxygen or indirectly by the action of organisms such as bacteria).
page 772 With a catalyst, the reaction is always faster, therefore costing less energy to run. In addition, with a catalyst the reaction may occur readily at a lower temperature, also costing less energy.
page 773 Fossil fuel combustion puts a great deal more CO2 in the atmosphere right now than any supercritical use of CO2. Compared to other (halogenated organic) solvents, supercritical CO2 is far less toxic to life. Therefore, at present, using CO2 as a solvent or a reactant in industrial processes is a reasonable choice for environmental sustainability.
page 774 Use room temperature and room pressure; use water as a solvent if possible; use O2 as the oxidizing agent instead of hydrogen peroxide if possible.
page 775 sp before reaction; sp2 after reaction
CHAPTER 19
page 787 No, nonspontaneous processes can occur so long as they receive some continuous outside assistance. Examples of nonspontaneous processes with which we may be familiar include the building of a brick wall and the electrolysis of water to form hydrogen gas and oxygen gas.
page 789 No. Just because the system is restored to its original condition doesn't mean that the surroundings have likewise been restored to their original condition, so it is not necessarily reversible.
page 791 ΔS depends not merely on q but on qrev. Although there are many possible paths that could take a system from its initial to final state, there is always only one reversible isothermal path between two states. Thus, ΔS has only one particular value regardless of the path taken between states.
page 793 Because rusting is a spontaneous process, ΔSuniv must be positive. Therefore, the entropy of the surroundings must increase, and that increase must be larger than the entropy decrease of the system.
page 795 S = 0, based on Equation 19.5 and the fact that ln 1 = 0.
page 796 A molecule can vibrate (atoms moving relative to one another) and rotate (tumble), whereas a single atom cannot undergo these motions.
page 799 It must be a perfect crystal at 0 K (third law of thermodynamics), which means it has only a single accessible microstate.
page 803 ΔSsurr always increases. For simplicity, assume that the process is isothermal. The change in entropy of the surroundings in an isothermal process is ![]() . Because the reaction is exothermic, –qsys is a positive number. Thus, ΔSsurr is a positive number and the entropy of the surroundings increases.
. Because the reaction is exothermic, –qsys is a positive number. Thus, ΔSsurr is a positive number and the entropy of the surroundings increases.
page 805 (a) In any spontaneous process the entropy of the universe increases. (b) In any spontaneous process operating at constant temperature, the free energy of the system decreases.
page 806 It indicates that the process to which the thermodynamic quantity refers has taken place under standard conditions, as summarized in Table 19.2.
page 810 Above the boiling point, vaporization is spontaneous, and ΔG < 0. Therefore, ΔH – TΔS < 0, and ΔH < TΔS.
CHAPTER 20
page 829 Oxygen is first assigned an oxidation number of – 2. Nitrogen must then have a + 3 oxidation number for the sum of oxidation numbers to equal – 1, the charge of the ion.
page 832 No. Electrons should appear in the two half–reactions but cancel when the half-reactions are added properly.
page 839 Yes. A redox reaction with a positive standard cell potential is spontaneous under standard conditions.
page 840 1 atm pressure of Cl2(g) and 1 M concentration of Cl–(aq)
page 846 Using data from Appendix E, we have ![]() = – 0.126 V for Pb2+ (aq)
= – 0.126 V for Pb2+ (aq) ![]() Pb(s) and
Pb(s) and ![]() = 0.854 V for Hg2+ (aq)
= 0.854 V for Hg2+ (aq) ![]() Hg(l). Because Pb(s) has the most negative value for E°red, it is the stronger reducing agent. (See Figure 20.12.) The comparison can also be made by reference to the activity series where Pb lies also above Hg, indicating that Pb is oxidized more readily than Hg. The more readily a substance is oxidized, the stronger it is as a reducing agent.
Hg(l). Because Pb(s) has the most negative value for E°red, it is the stronger reducing agent. (See Figure 20.12.) The comparison can also be made by reference to the activity series where Pb lies also above Hg, indicating that Pb is oxidized more readily than Hg. The more readily a substance is oxidized, the stronger it is as a reducing agent.
page 859 Al, Zn. Both are easier to oxidize than Fe.
CHAPTER 21
page 877 The mass number decreases by 4.
page 879 Only the neutron, as it is the only neutral particle listed.
page 883 From Figure 21.4 we can see that each of these four elements has only one stable isotope, and from their atomic numbers we see that they each have an odd number of protons. Given the rarity of stable isotopes with odd numbers of neutrons and protons, we expect that each isotope will possess an even number of neutrons. From their atomic weights we see that this is the case: F (10 neutrons), Na (12 neutrons), Al (14 neutrons), and P (16 neutrons).
page 885 No. Electric and magnetic fields are only effective at accelerating charged particles and a neutron is not charged.
page 889 top Spontaneous radioactive decay is a unimolecular process: A ![]() Products. The rate law that fits this observation is a first-order kinetic rate law, rate = k[A]. A second-order kinetic process has rate = k[A]2 and the elementary reaction is bimolecular: A + A
Products. The rate law that fits this observation is a first-order kinetic rate law, rate = k[A]. A second-order kinetic process has rate = k[A]2 and the elementary reaction is bimolecular: A + A ![]() Products. A zero-order kinetic process has = k, and the rate does not change until the limiting reactant is entirely consumed. The latter two rate laws do not fit a unimolecular process.
Products. A zero-order kinetic process has = k, and the rate does not change until the limiting reactant is entirely consumed. The latter two rate laws do not fit a unimolecular process.
page 889 (bottom) (a) Yes; doubling the mass would double the amount of radioactivity of the sample as shown in Equation 21.18. (b) No; changing the mass would not change the half-life as shown in Equation 21.20.
page 892 No. Alpha particles are more readily absorbed by matter than beta or gamma rays. Geiger counters must be calibrated for the radiation they are being used to detect.
page 896 (top) The values in Table 21.7 only reflect the mass of the nucleus, while the atomic mass is the sum of the mass of the nucleus and the electrons. So the atomic mass of iron-56 is 26 × me larger than the nuclear mass.
page 896 (bottom) No. Stable nuclei having mass numbers around 100 are the most stable nuclei. They could not form a still more stable nucleus with an accompanying release of energy.
page 905 The absorbed dose is equal to 0.10 J × (1 rad/1 × 10–2 J) = 10 rads. The effective dosage is calculated by multiplying the absorbed dose by the relative biological effectiveness (RBE) factor, which is 10 for alpha radiation. Thus, the effective dosage is 100 rems.
CHAPTER 22
page 919 (top) No.
page 919 No. N can form triple bonds but P cannot, as it would have to form P2.
page 921 H–, hydride.
page 923 +1 for everything except H2, for which the oxidation state of H is 0
page 924 No—it is the volume of Pd that can increase to accommodate hydrogen, not its mass.
page 927 0 for Cl2; –1 for Cl–; +1 for ClO–
page 929 They should both be strong, since the central halogen is in the +5 oxidation state for both of them. We need to look up the redox potentials to see which ion, BrO3– or ClO3–, has the larger reduction potential. The ion with the larger reduction potential is the stronger oxidizing agent. BrO3– is the stronger oxidizing agent on this basis (+1.52 V standard reduction potential in acid compared to +1.47 V for ClO3–).
page 931 The standard energy to dissociate one mole of oxygen atoms from one mole of ozone was given as 105 kJ. If we assume, as usual, that one photon will dissociate one molecule, that means the energy of the photons should be 105 kJ per mole (of photons). Using Avogadro's number, we can calculate that one photon would then have 1.744 × 10–19 J of energy. Using equations from Chapter 6, c = λv and E = hv, we can find that a photon with 1.744 × 10–19 J of energy will have a wavelength λ of 1140 nm, or 1.14 × 106 m, which is in the infrared part of the spectrum.
page 932 HIO3
page 936 SO3(g) + H2O(l) ![]() H2SO4(l)
H2SO4(l)
page 940 (a) + 5 (b) + 3
page 948 CO2(g)
page 949 Yes, it must, since CO2 is a liquid at room temperature and pressure, and CO2 is a gas.
page 952 Silicon is the element, Si. Silica is SiO2. Silicones are polymers that have an O — Si — O backbone and hydrocarbon groups on the Si.
page 953 +3
CHAPTER 23
page 965 Sc is the biggest.
page 967 You would have to remove core electrons.
page 968 (top) The larger the distance, the weaker the spin-spin interactions.
page 968 (bottom) Yes, it is a Lewis acid–base interaction; the metal ion is the Lewis acid (electron pair acceptor).
page 972 [Fe(H2O)6]3+ (aq) + SCN–(aq) ![]() [Fe(H2O)5SCN]2+ (aq) + H2O(l)
[Fe(H2O)5SCN]2+ (aq) + H2O(l)
page 974 (a) tetrahedral (b) octahedral
page 976 Bidentate
page 978 Its conjugation (alternating single and double CC bonds)
page 981 No, ammonia cannot engage in linkage isomerism—the only atom that can coordinate to a metal is the nitrogen.
page 985 Both isomers have the same chemical formulas and the same donor atoms on the ligands bonding to the metal ion. The difference is that the d isomer has a right-handed “twist” and the l isomer has a “left-handed” twist.
page 987 Co is 1s2 2s2 2p6 3s2 3p6 4s2 3d7. Co3+ is 1s2 2s2 2p6 3s2 3p6 3d6. Co has 3 unpaired electrons; Co3+ has 4 unpaired electrons, assuming all 5 d orbitals have the same energy.
page 989 It has lost all of the Ti valence electrons; only core electrons remain, and the energy gap between filled and empty orbitals is large, corresponding to light in the ultraviolet, which we cannot perceive as colored.
page 991 Low spin
page 992 The ligands are in the xy plane. The dxy orbital has its lobes mostly in that plane, so its energy is higher than dxz and dyz.
CHAPTER 24
page 1007 C ═ N, because it is a polar double bond. C — H and C — C bonds are relatively unreactive.
page 1009 Two C — H bonds and two C — C bonds
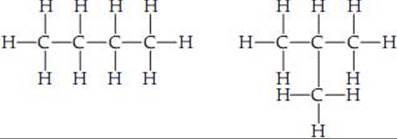
page 1010 The isomers have different properties, as seen in Table 24.3.
page 1015 Only two of the four possible C ═ C bond sites are distinctly different in the linear chain of five carbon atoms with one double bond.
page 1021
page 1025
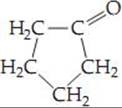
page 1029 All four groups must be different from one another.
page 1033 No. Breaking the hydrogen bonds between N — H and O ═ C groups in a protein by heating causes the α-helix structure to unwind and the β-sheet structure to separate.
page 1037 The α form of the C — O — C linkage. Glycogen serves as a source of energy in the body, which means that the body's enzymes must be able to hydrolyze it to sugars. The enzymes work only on polysaccharides having the α linkage.