CHEMISTRY THE CENTRAL SCIENCE
5 THERMOCHEMISTRY
5.4 ENTHALPIES OF REACTION
Because ΔH = Hfinal —Hinitial, the enthalpy change for a chemical reaction is given by
![]()
The enthalpy change that accompanies a reaction is called either the enthalpy of reaction or the heat of reaction and is sometimes written ΔHrxn, where “rxn” is a commonly used abbreviation for “reaction.”
When we give a numerical value for ΔHrxn, we must specify the reaction involved. For example, when 2 mol H2(g) burn to form 2 mol H2O(g) at a constant pressure, the system releases 483.6 kJ of heat. We can summarize this information as
![]()
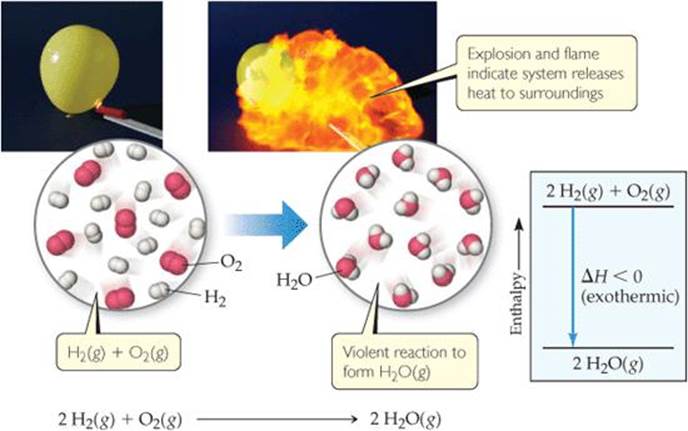
![]() FIGURE 5.14 Exothermic reaction of hydrogen with oxygen. When a mixture of H2(g) and O2(g) is ignited to form H2O(g), the resultant explosion produces a ball of flame. Because the system releases heat to the surroundings, the reaction is exothermic as indicated in the enthalpy diagram.
FIGURE 5.14 Exothermic reaction of hydrogen with oxygen. When a mixture of H2(g) and O2(g) is ignited to form H2O(g), the resultant explosion produces a ball of flame. Because the system releases heat to the surroundings, the reaction is exothermic as indicated in the enthalpy diagram.
The negative sign for ΔH tells us that this reaction is exothermic. Notice that ΔH is reported at the end of the balanced equation, without explicitly specifying the amounts of chemicals involved. In such cases the coefficients in the balanced equation represent the number of moles of reactants and products producing the associated enthalpy change. Balanced chemical equations that show the associated enthalpy change in this way are called thermochemical equations.
The exothermic nature of this reaction is also shown in the enthalpy diagram in ![]() FIGURE 5.14. Notice that the enthalpy of the reactants is greater (more positive) than the enthalpy of the products. Thus, ΔH = Hproducts – Hreactants is negative.
FIGURE 5.14. Notice that the enthalpy of the reactants is greater (more positive) than the enthalpy of the products. Thus, ΔH = Hproducts – Hreactants is negative.
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
If the reaction to form water were written ![]() , would you expect the same value of ΔH as in Equation 5.17? Why or why not?
, would you expect the same value of ΔH as in Equation 5.17? Why or why not?
The reaction of hydrogen with oxygen is highly exothermic and occurs rapidly once it starts. It can occur with explosive violence, as demonstrated by the explosions of the German airship Hindenburg in 1937 (![]() FIGURE 5.15) and the U.S. space shuttle Challenger in 1986.
FIGURE 5.15) and the U.S. space shuttle Challenger in 1986.

![]() FIGURE 5.15 The burning of the hydrogen-filled airship Hindenburg. This tragedy, in Lakehurst, New Jersey, on May 6, 1937, led to the discontinuation of hydrogen as a buoyant gas in such craft. Modern-day airships are filled with helium, which is not as buoyant as hydrogen but is not flammable.
FIGURE 5.15 The burning of the hydrogen-filled airship Hindenburg. This tragedy, in Lakehurst, New Jersey, on May 6, 1937, led to the discontinuation of hydrogen as a buoyant gas in such craft. Modern-day airships are filled with helium, which is not as buoyant as hydrogen but is not flammable.
The following guidelines are helpful when using thermochemical equations and enthalpy diagrams:
1. Enthalpy is an extensive property. The magnitude of ΔH is proportional to the amount of reactant consumed in the process. For example, 890 kJ of heat is pro duced when 1 mol of CH4 is burned in a constant-pressure system:
![]()
Because the combustion of 1 mol of CH4 with 2 mol of O2 releases 890 kJ of heat, the combustion of 2 mol of CH4 with 4 mol of O2 releases twice as much heat, 1780 kJ.
2. The enthalpy change for a reaction is equal in magnitude, but opposite in sign, to ΔH for the reverse reaction. For example, ΔH for the reverse of Equation 5.18 is +890 kJ:
![]()
When we reverse a reaction, we reverse the roles of the products and the reactants. From Equation 5.16, we see that reversing the products and reactants leads to the same magnitude of ΔH but a change in sign (![]() FIGURE 5.16).
FIGURE 5.16).
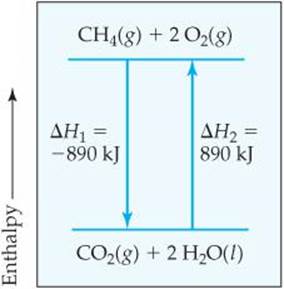
![]() FIGURE 5.16 ΔH for a reverse reaction. Reversing a reaction changes the sign but not the magnitude of the enthalpy change: ΔH2 = −ΔH1
FIGURE 5.16 ΔH for a reverse reaction. Reversing a reaction changes the sign but not the magnitude of the enthalpy change: ΔH2 = −ΔH1
3. The enthalpy change for a reaction depends on the states of the reactants and products. If the product in Equation 5.18 were H2O(g) instead of H2O(l), ΔH rxn would be –802 kJ instead of —890 kJ. Less heat would be available for transfer to the surroundings because the enthalpy of H2O(g) is greater than that of H2O(l). One way to see this is to imagine that the product is initially liquid water. The liquid water must be converted to water vapor, and the conversion of 2 mol H2O(l) to 2 mol H2O(g) is an endothermic process that absorbs 88 kJ:
![]()
Thus, it is important to specify the states of the reactants and products in thermochemical equations. In addition, we will generally assume that the reactants and products are both at the same temperature, 25 °C, unless otherwise indicated.
SAMPLE EXERCISE 5.4 Relating ΔH to Quantities of Reactants and Products
How much heat is released when 4.50 g of methane gas is burned in a constant-pressure system? (Use the information given in Equation 5.18.)
SOLUTION
Analyze Our goal is to use a thermochemical equation to calculate the heat produced when a specific amount of methane gas is combusted. According to Equation 5.18, 890 kJ is released by the system when 1 mol CH4 is burned at constant pressure.
Plan Equation 5.18 provides us with a stoichiometric conversion factor: (1 mol CH4![]() –890 kJ). Thus, we can convert moles of CH4 to kJ of energy. First, however, we must convert grams of CH4 to moles of CH4. Thus, the conversion sequence is grams CH4 (given) → moles CH4 → kJ (unknown to be found).
–890 kJ). Thus, we can convert moles of CH4 to kJ of energy. First, however, we must convert grams of CH4 to moles of CH4. Thus, the conversion sequence is grams CH4 (given) → moles CH4 → kJ (unknown to be found).
Solve By adding the atomic weights of C and 4 H, we have 1 mol CH4 = 16.0 CH4. We can use the appropriate conversion factors to convert grams of CH4 to moles of CH4 to kilojoules:
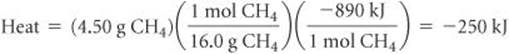
The negative sign indicates that the system released 250 kJ into the surroundings.
PRACTICE EXERCISE
Hydrogen peroxide can decompose to water and oxygen by the reaction
![]()
Calculate the quantity of heat released when 5.00 g of H2O2(l) decomposes at constant pressure.
Answer: –14.4 kJ
 STARTEGIES IN CHEMISTRY
STARTEGIES IN CHEMISTRY
USING ENTHALPY AS A GUIDE
If you hold a brick in the air and let it go, you know what happens: It falls as the force of gravity pulls it toward Earth. A process that is thermodynamically favored to happen, such as a brick falling to the ground, is called a spontaneous process. A spontaneous process can be either fast or slow; the rate at which processes occur is not governed by thermodynamics.
Chemical processes can be thermodynamically favored, or spontaneous, too. By spontaneous, however, we do not mean that the reaction will form products without any intervention. That can be the case, but often some energy must be imparted to get the process started. The enthalpy change in a reaction gives one indication as to whether the reaction is likely to be spontaneous. The combustion of H2(g) and O2(g), for example, is highly exothermic:
![]()
Hydrogen gas and oxygen gas can exist together in a volume indefinitely without noticeable reaction occurring. Once the reaction is initiated, however, energy is rapidly transferred from the system (the reactants) to the surroundings as heat. The system thus loses enthalpy by transferring the heat to the surroundings. (Recall that the first law of thermodynamics tells us that the total energy of the system plus the surroundings does not change; energy is conserved.)
Enthalpy change is not the only consideration in the spontaneity of reactions, however, nor is it a foolproof guide. For example, even though ice melting is an endothermic process,
![]()
this process is spontaneous at temperatures above the freezing point of water (0 °C). The reverse process, water freezing, is spontaneous at temperatures below 0 °C. Thus, we know that ice at room temperature melts and water put into a freezer at –20 °C turns into ice. Both processes are spontaneous under different conditions even though they are the reverse of one another. In Chapter 19 we will address the spontaneity of processes more fully. We will see why a process can be spontaneous at one temperature but not at another, as is the case for the conversion of water to ice.
Despite these complicating factors, you should pay attention to the enthalpy changes in reactions. As a general observation, when the enthalpy change is large, it is the dominant factor in determining spontaneity. Thus, reactions for which ΔH is large and negative tend to be spontaneous. Reactions for which ΔH is large and positive tend to be spontaneous only in the reverse direction.
RELATED EXERCISES: 5.47, 5.48
In many situations we will find it valuable to know the sign and magnitude of the enthalpy change associated with a given chemical process. As we see in the following sections, ΔH can be either determined directly by experiment or calculated from known enthalpy changes of other reactions.