CHEMISTRY THE CENTRAL SCIENCE
5 THERMOCHEMISTRY
5.8 FOODS AND FUELS
Most chemical reactions used for the production of heat are combustion reactions. The energy released when one gram of any substance is combusted is the fuel value of the substance. The fuel value of any food or fuel can be measured by calorimetry.
Foods
Most of the energy our bodies need comes from carbohydrates and fats. The carbohydrates known as starches are decomposed in the intestines into glucose, C6H12O6. Glucose is soluble in blood, and in the human body it is known as blood sugar. It is transported by the blood to cells where it reacts with O2 in a series of steps, eventually producing CO2(g), H2O(l), and energy:
![]()
Because carbohydrates break down rapidly, their energy is quickly supplied to the body. However, the body stores only a very small amount of carbohydrates. The average fuel value of carbohydrates is 17 kJ/g (4 kcal/g).*
Like carbohydrates, fats produce CO2 and H2O when metabolized. The reaction of tristearin, C57H110O6, a typical fat, is
![]()
The body uses the chemical energy from foods to maintain body temperature (see the “Chemistry and Life” box in Section 5.5), to contract muscles, and to construct and repair tissues. Any excess energy is stored as fats. Fats are well suited to serve as the body's energy reserve for at least two reasons: (1) They are insoluble in water, which facilitates storage in the body, and (2) they produce more energy per gram than either proteins or carbohydrates, which makes them efficient energy sources on a mass basis. The average fuel value of fats is 38 kJ/g (9 kcal/g).
The combustion of carbohydrates and fats in a bomb calorimeter gives the same products as when they are metabolized in the body. The metabolism of proteins produces less energy than combustion in a calorimeter because the products are different. Proteins contain nitrogen, which is released in the bomb calorimeter as N2. In the body this nitrogen ends up mainly as urea, (NH2)2CO. Proteins are used by the body mainly as building materials for organ walls, skin, hair, muscle, and so forth. On average, the metabolism of proteins produces 17 kJ/g (4 kcal/g), the same as for carbohydrates.
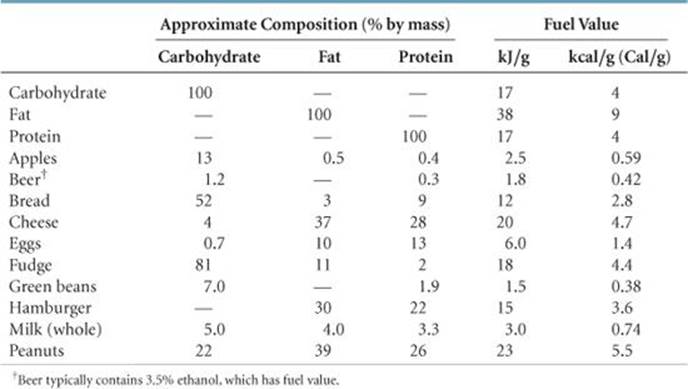
Fuel values for some common foods are shown in ![]() TABLE 5.4. Labels on packaged foods show the amounts of carbohydrate, fat, and protein contained in an average serving, as well as the amount of energy supplied by a serving (
TABLE 5.4. Labels on packaged foods show the amounts of carbohydrate, fat, and protein contained in an average serving, as well as the amount of energy supplied by a serving (![]() FIGURE 5.24).
FIGURE 5.24).
![]() GO FIGURE
GO FIGURE
Which value would change most if this label were for skim milk instead of whole milk: grams of fat, grams of total carbohydrate, or grams of protein?
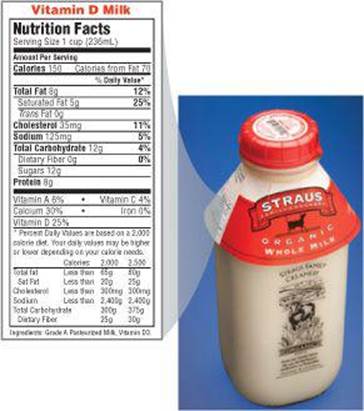
![]() FIGURE 5.24 Nutrition label for whole milk.
FIGURE 5.24 Nutrition label for whole milk.
TABLE 5.4 • Compositions and Fuel Values of Some Common Foods
The amount of energy our bodies require varies considerably, depending on such factors as weight, age, and muscular activity. About 100 kJ per kilogram of body mass per day is required to keep the body functioning at a minimal level. An average 70-kg (154-lb) person expends about 800 kJ/hr when doing light work, and strenuous activity often requires 2000 kJ/hr or more. When the fuel value, or caloric content, of the food we ingest exceeds the energy we expend, our body stores the surplus as fat.
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
Which releases the greatest amount of energy per gram when metabolized: carbohydrates, proteins, or fats?
SAMPLE EXERCISE 5.13 Comparing Fuel Values
Celery contains carbohydrates in the form of starch and cellulose, which have essentially the same fuel values when combusted in a bomb calorimeter. When we eat celery, however, our bodies receive fuel value from the starch only. What can we conclude about the difference between starch and cellulose as foods?
SOLUTION
If cellulose does not provide fuel value, we must conclude that it is not converted in the body into CO2 and H2O, as starch is. A slight but critical difference in the structures of starch and cellulose explains why only starch is broken down into glucose in the body. Cellulose passes through without undergoing significant chemical change. It serves as fiber, or roughage, in the diet but provides no caloric value.
PRACTICE EXERCISE
The nutrition label on a bottle of canola oil indicates that 10 g of the oil has a fuel value of 86 kcal. A similar label on a bottle of pancake syrup indicates that 60 mL (about 60 g) has a fuel value of 200 kcal. Account for the difference.
Answer: The oil has a fuel value of 8.6 kcal/g, whereas the syrup has a fuel value of about 3.3 kcal/g. The higher fuel value for the canola oil arises because the oil is essentially pure fat, whereas the syrup is a solution of sugars (carbohydrates) in water. The oil has a higher fuel value per gram; in addition, the syrup is diluted by water.
SAMPLE EXERCISE 5.14 Estimating the Fuel Value of a Food from Its Composition
(a) A 28-g (1-oz) serving of a popular breakfast cereal served with 120 mL of skim milk provides 8 g protein, 26 g carbohydrates, and 2 g fat. Using the average fuel values of these substances, estimate the fuel value (caloric content) of this serving. (b) A person of average weight uses about 100 Cal/mi when running or jogging. How many servings of this cereal provide the fuel value requirements to run 3 mi?
SOLUTION
(a) Analyze The fuel value of the serving will be the sum of the fuel values of the protein, carbohydrates, and fat.
Plan We are given the masses of the protein, carbohydrates, and fat contained in a serving. We can use the data in Table 5.4 to convert these masses to their fuel values, which we can sum to get the total fuel value.
Solve
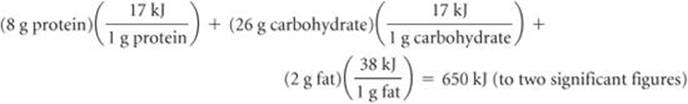
This corresponds to 160 kcal:
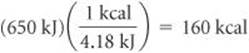
Recall that the dietary Calorie is equivalent to 1 kcal. Thus, the serving provides 160 Cal.
(b) Analyze Here we are faced with the reverse problem, calculating the quantity of food that provides a specific fuel value.
Plan The problem statement provides a conversion factor between Calories and miles. The answer to part (a) provides us with a conversion factor between servings and Calories.
Solve We can use these factors in a straightforward dimensional analysis to determine the number of servings needed, rounded to the nearest whole number:
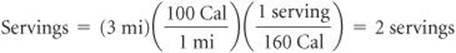
PRACTICE EXERCISE
(a) Dry red beans contain 62% carbohydrate, 22% protein, and 1.5% fat. Estimate the fuel value of these beans. (b) During a very light activity, such as reading or watching television, the average adult expends about 7 kJ/min. How many minutes of such activity can be sustained by the energy provided by a serving of chicken noodle soup containing 13 g protein, 15 g carbohydrate, and 5 g fat?
Answers: (a) 15 kJ/g, (b) 100 min
Fuels
During the complete combustion of fuels, carbon is converted to CO2 and hydrogen is converted to H2O, both of which have large negative enthalpies of formation. Consequently, the greater the percentage of carbon and hydrogen in a fuel, the higher its fuel value. In ![]() TABLE 5.5, for example, compare the compositions and fuel values of bituminous coal and wood. The coal has a higher fuel value because of its greater carbon content.
TABLE 5.5, for example, compare the compositions and fuel values of bituminous coal and wood. The coal has a higher fuel value because of its greater carbon content.
In 2008 the United States consumed 1.05 × 1017 kJ of energy. This value corresponds to an average daily energy consumption per person of 9.4 × 105 kJ, roughly 100 times greater than the per capita food-energy needs. Although the population of the United States is only about 4.5% of the world's population, the United States accounts for nearly 20% of the world's total energy consumption. ![]() FIGURE 5.25 illustrates the sources of this energy.
FIGURE 5.25 illustrates the sources of this energy.
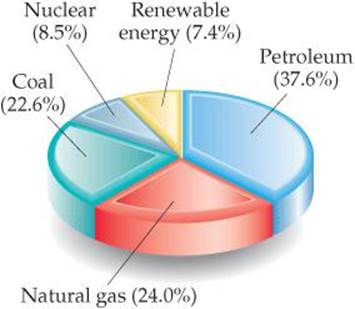
![]() FIGURE 5.25 Energy consumption in the United States. In 2008 the United States consumed a total of 1.05 × 1017 kJ of energy.
FIGURE 5.25 Energy consumption in the United States. In 2008 the United States consumed a total of 1.05 × 1017 kJ of energy.
Coal, petroleum, and natural gas, which are the world's major sources of energy, are known as fossil fuels. All have formed over millions of years from the decomposition of plants and animals and are being depleted far more rapidly than they are being formed.
Natural gas consists of gaseous hydrocarbons, compounds of hydrogen and carbon. It contains primarily methane (CH4), with small amounts of ethane (C2H6), propane (C3H8), and butane (C4H10). We determined the fuel value of propane in Sample Exercise 5.11. Petroleum is a liquid composed of hundreds of compounds, most of which are hydrocarbons, with the remainder being chiefly organic compounds containing sulfur, nitrogen, or oxygen. Coal, which is solid, contains hydrocarbons of high molecular weight as well as compounds containing sulfur, oxygen, or nitrogen. Coal is the most abundant fossil fuel; current reserves are projected to last for well over 100 years at current consumption rates. However, the use of coal presents a number of problems.
TABLE 5.5 • Fuel Values and Compositions of Some Common Fuels
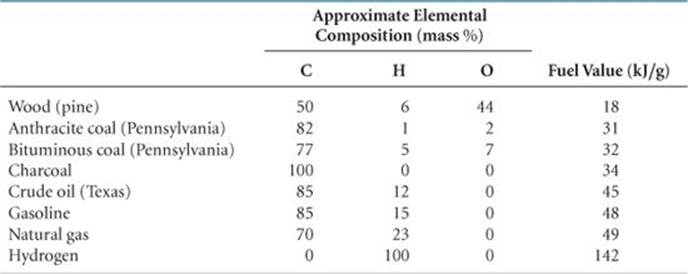
Coal is a complex mixture of substances, and it contains components that cause air pollution. When coal is combusted, the sulfur it contains is converted mainly to sulfur dioxide, SO2, a troublesome air pollutant. Because coal is a solid, recovery from its underground deposits is expensive and often dangerous. Furthermore, coal deposits are not always close to locations of high-energy use, so there are often substantial shipping costs.
Fossil fuels release energy in combustion reactions, which ideally produce only CO2 and H2O. The production of CO2 has become a major issue that involves science and public policy because of concerns that increasing concentrations of atmospheric CO2 are causing global climate changes. In December 2009 the United Nations held a Climate Change Conference in Copenhagen, Denmark, that attracted about 15,000 participants from nearly 200 countries, including many government leaders. Much of the discussion at this conference involved the impact of atmospheric CO2 and ways in which alternative energy sources could be implemented. We will discuss the environmental aspects of atmospheric CO2 in Chapter 18.
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
Much current research is directed toward using hydrogen gas, H2(g), as a fuel. What intrinsic advantage does hydrogen as a fuel have with respect to the current climate-change debate?
Other Energy Sources
Nuclear energy is the energy released in either the splitting or the fusion (combining) of atomic nuclei. Nuclear power is currently used to produce about 21% of the electric power in the United States and makes up about 8.5% of the total U.S. energy production (Figure 5.25). Nuclear energy is, in principle, free of the polluting emissions that are a major problem with fossil fuels. However, nuclear power plants produce radioactive waste products, and their use has therefore been controversial. We will discuss issues related to the production of nuclear energy in Chapter 21.
Fossil fuels and nuclear energy are nonrenewable sources of energy—they are limited resources that we are consuming at a much greater rate than they can be regenerated. Eventually these fuels will be expended, although estimates vary greatly as to when this will occur. Because nonrenewable energy sources will eventually be used up, a great deal of research is being conducted on renewable energy sources, sources that are essentially inexhaustible. Renewable energy sources include solar energy from the Sun, wind energy harnessed by windmills, geothermal energy from the heat stored inside Earth, hydroelectric energy from flowing rivers, and biomass energy from crops and biological waste matter. Currently, renewable sources provide about 7.4% of the U.S. annual energy consumption, with hydroelectric and biomass sources the major contributors.
Fulfilling our future energy needs will depend on developing technology to harness solar energy with greater efficiency. Solar energy is the world's largest energy source. On a clear day about 1 kJ of solar energy reaches each square meter of Earth's surface every second. The average solar energy falling on only 0.1% of U.S. land area is equivalent to all the energy this nation currently uses. Harnessing this energy is difficult because it is dilute (that is, distributed over a wide area) and varies with time of day and weather conditions. The effective use of solar energy will depend on the development of some means of storing and distributing it. Any practical means for doing this will almost certainly involve an endothermic chemical process that can be later reversed to release heat. One such reaction is
![]()
This reaction proceeds in the forward direction at high temperatures, which can be obtained in a solar furnace. The CO and H2 formed in the reaction could then be stored and allowed to react later, with the heat released being put to useful work.
 CHEMISTRY PUT TO WORK
CHEMISTRY PUT TO WORK
The Scientific and Political Challenges of Biofuels
One of the biggest challenges facing us in the twenty-first century is production of abundant sources of energy, both food and fuels. World population more than doubled from 1960 to 2000, from about 3 billion to more than 6 billion people. It continues to grow at a rate of about 750 million per decade—at the end of 2009, the global population was about 6.8 billion people. A growing world population puts greater demands on the global food supply, especially in Asia and Africa, which together make up more than 75% of the world population.
A growing population also increases demands on the production of fuels for transportation, industry, electricity, heating, and cooling. Further, many of the most populous nations, such as China and India, have seen dramatic increases in the quality of life among their citizens. As these countries have modernized, their per capita consumption of energy—for automobiles, new industries, modern housing, and technology advances—has increased significantly. In China, for instance, per capita energy consumption roughly doubled between 1990 and 2010 (although it is still less than 20% of U.S. per capita energy consumption).
Global fuel energy consumption in 2009 was more than 5 × 1017 kJ, a staggeringly large number that is projected to grow to more than 7 × 1017 kJ by 2030. More than 80% of current energy requirements comes from combustion of nonrenewable fossil fuels, especially petroleum. Depletion has generally increased the cost of petroleum-based fuels. In addition, the exploration of new petroleum sources often involves environmentally sensitive regions, such as the Arctic National Wildlife Refuge. Thus, increasing the supplies of petroleum becomes a major political and economic issue.
Global dependence on petroleum is in large part because it provides liquid fuels, such as gasoline, that are critical to supplying transportation needs. One of the most promising––but controversial––alternatives to petroleum-based fuels is biofuels, liquid fuels derived from biological matter. The most common approach to producing biofuels is to transform plant sugars and other carbohydrates into combustible liquids. The energy stored in the carbohydrates produced in photosynthesis (Equation 5.32) is higher than the energy in H2O and CO2; thus, photosynthesis is a way to “store” solar energy in plants.
The most commonly produced biofuel is bioethanol, which is ethanol (C2H5OH) made from fermentation of plant carbohydrates. The fuel value of ethanol is about two-thirds that of gasoline and is therefore comparable to that of coal (Table 5.5). The United States and Brazil dominate bioethanol production, together supplying 85% of the world's total.
In the United States, nearly all the bioethanol currently produced is made from yellow feed corn (![]() FIGURE 5.26). Glucose (C6H12O6) in the corn is converted to ethanol and CO2:
FIGURE 5.26). Glucose (C6H12O6) in the corn is converted to ethanol and CO2:
![]()
Notice that this reaction is anaerobic—it does not involve O2(g)—and that the enthalpy change is positive and much smaller in magnitude than for most combustion reactions. Other carbohydrates can be converted to ethanol in similar fashion.
Producing bioethanol from corn is controversial for two main reasons. First, growing and transporting corn are both energy-intensive processes, and growing it requires the use of fertilizers. It is estimated that the energy return on corn-based bioethanol is only 34%—that is, for each 1.00 J of energy expended to produce the corn, 1.34 J of energy is produced in the form of bioethanol. Second, the use of corn as a starting material for making bioethanol competes with its use as an important component of the food chain (the so-called “food versus fuel” debate). In particular, the diversion of corn crops to bioethanol production has led to higher prices for food, including beef (corn is used as feed for cattle). Much current research focuses on the formation of bioethanol from cellulosic plants, plants that contain the complex carbohydrate cellulose. Cellulose is not readily metabolized (Sample Exercise 5.13) and so does not compete with the food supply. However, the chemistry for converting cellulose to ethanol is much more complex than that for converting corn. Cellulosic bioethanol could be produced from very fast growing nonfood plants, such as prairie grasses and switchgrass, which readily renew themselves without the use of fertilizers.
As shown in the chapter-opening photograph, the Brazilian bioethanol industry uses sugarcane as its feedstock. Sugarcane grows much faster than corn and without the need for fertilizers or tending. Because of these differences, the energy return for sugarcane is much higher than the energy return for corn. It is estimated that for each 1.0 J of energy expended in growing and processing sugarcane, 8.0 J of energy is produced as bioethanol. Because the climate in Brazil is ideal for growing cane, the Brazilian government started investing in the 1970s in ways to utilize sugarcane as a major fuel source.
Other biofuels that are also becoming a major part of the world economy include biodiesel, a substitute for petroleum-derived diesel fuel. Biodiesel is typically produced from crops that have a high oil content, such as soybeans and canola. It can also be produced from animal fats and waste vegetable oil from the food and restaurant industry.
Biofuels are combusted in the presence of O2(g) to produce CO2(g), H2O(g), and energy in the form of heat, much as hydrocarbon fuels do. Because CO2(g) is a product, the use of biofuels is part of the international debate about carbon dioxide and climate change. We will discuss this issue further in Chapter 18.

![]() FIGURE 5.26 Corn, a source of food and bioethanol. The sugars in the kernels of feed corn can be used as food or as a feedstock for fermentation to ethanol.
FIGURE 5.26 Corn, a source of food and bioethanol. The sugars in the kernels of feed corn can be used as food or as a feedstock for fermentation to ethanol.
RELATED EXERCISES: 5.89, 5.90, 5.111, 5.119
Plants utilize solar energy in photosynthesis, the reaction in which the energy of sunlight is used to convert CO2 and H2O into carbohydrates and O2. One common pho-tosynthetic reaction produces the sugar glucose:
![]()
In essence, photosynthesis is the reverse of combustion in that CO2 and H2O are consumed and O2 and an organic molecule are produced. Photosynthesis is an important part of Earth's ecosystem because it replenishes atmospheric O2, produces an energy-rich molecule that can be used as fuel, and consumes some atmospheric CO2.
Perhaps the most direct way to use the Sun's energy is to convert it directly into electricity in photovoltaic devices, sometimes called solar cells. The efficiencies of such devices have increased dramatically during the past few years. Photovoltaics are vital to the generation of power for space vehicles, such as the International Space Station research facility currently orbiting Earth. Technological advances have led to solar panels that last longer and produce electricity with greater efficiency at steadily decreasing unit cost. Indeed, the future of solar energy is, like the Sun itself, very bright.
SAMPLE INTEGRATIVE EXERCISE Putting Concepts Together
Trinitroglycerin, C3H5N3O9 (usually referred to simply as nitroglycerin), has been widely used as an explosive. Alfred Nobel used it to make dynamite in 1866. Rather surprisingly, it also is used as a medication, to relieve angina (chest pains resulting from partially blocked arteries to the heart) by dilating the blood vessels. At 1 atm pressure and 25 °C, the enthalpy of decomposition of trinitroglycerin to form nitrogen gas, carbon dioxide gas, liquid water, and oxygen gas is–1541.4 kJ/mol. (a) Write a balanced chemical equation for the decomposition of triniW r i t e a balanced troglycerin. (b) Calculate the standard heat of formation of trinitroglycerin. (c) A standard dose of trinitroglycerin for relief of angina is 0.60 mg. If the sample is eventually oxidized in the body (not explosively, though!) to nitrogen gas, carbon dioxide gas, and liquid water, what number of calories is released? (d) One common form of trinitroglycerin melts at about 3 °C. From this information and the formula for the substance, would you expect it to be a molecular or ionic compound? Explain. (e) Describe the various conversions of forms of energy when trinitroglycerin is used as an explosive to break rockfaces in highway construction.
SOLUTION
(a) The general form of the equation we must balance is
C3H5N3O9(l) ← N2(g) + CO2(g) + H2O(l) + O2(g)
We go about balancing in the usual way. To obtain an even number of nitrogen atoms on the left, we multiply the formula for C3H5N3O9 by 2, which gives us 3 mol of N2, 6 mol of CO2 and 5 mol of H2O. Everything is balanced except for oxygen. We have an odd number of oxygen atoms on the right. We can balance the oxygen by adding ![]() mol of O2 on the right:
mol of O2 on the right:
![]()
We multiply through by 2 to convert all coefficients to whole numbers:
![]()
(At the temperature of the explosion, water is a gas. The rapid expansion of the gaseous products creates the force of an explosion.)
(b) The heat of formation is the enthalpy change in the balanced chemical equation:
![]()
We can obtain the value of ![]() by using the equation for the heat of decomposition of trinitroglycerin:
by using the equation for the heat of decomposition of trinitroglycerin:
![]()
The enthalpy change in this reaction is 4(−1541.4 kJ) = −6165.6 kJ. [We need to multiply by 4 because there are 4 mol of C3H5N3O9(l) in the balanced equation.] This enthalpy change is given by the sum of the heats of formation of the products minus the heats of formation of the reactants, each multiplied by its coefficient in the balanced equation:
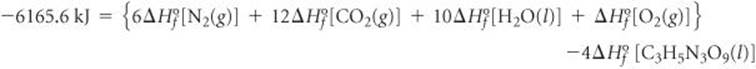
The ![]() values for N2(g) and O2(g) are zero, by definition. We look up the values for H2O(l) and CO2(g) in Table 5.3 and find that
values for N2(g) and O2(g) are zero, by definition. We look up the values for H2O(l) and CO2(g) in Table 5.3 and find that
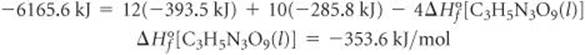
(c) We know that on oxidation 1 mol of C3H5N3O9(l) yields 1541.4 kJ. We need to calculate the number of moles of C3H5N3O9(l) in 0.60 mg:
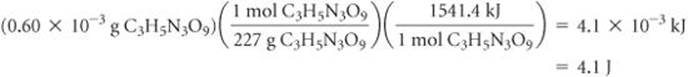
(d) Because trinitroglycerin melts below room temperature, we expect that it is a molecular compound. With few exceptions, ionic substances are generally hard, crystalline materials that melt at high temperatures. ![]() (Sections 2.6 and 2.7) Also, the molecular formula suggests that it is likely to be a molecular substance. All the elements of which it is composed are nonmetals.
(Sections 2.6 and 2.7) Also, the molecular formula suggests that it is likely to be a molecular substance. All the elements of which it is composed are nonmetals.
(e) The energy stored in trinitroglycerin is chemical potential energy. When the substance reacts explosively, it forms substances such as carbon dioxide, water, and nitrogen gas, which are of lower potential energy. In the course of the chemical transformation, energy is released in the form of heat; the gaseous reaction products are very hot. This very high heat energy is transferred to the surroundings; the gases expand against the surroundings, which may be solid materials. Work is done in moving the solid materials and imparting kinetic energy to them. For example, a chunk of rock might be impelled upward. It has been given kinetic energy by transfer of energy from the hot, expanding gases. As the rock rises, its kinetic energy is transformed into potential energy. Eventually, it again acquires kinetic energy as it falls to Earth. When it strikes Earth, its kinetic energy is converted largely to thermal energy, though some work may be done on the surroundings as well.