CHEMISTRY THE CENTRAL SCIENCE
6 ELECTRONIC STRUCTURE OF ATOMS
EXERCISES
VISUALIZING CONCEPTS
6.1 Consider the water wave shown here. (a) How could you measure the speed of this wave? (b) How would you determine the wavelength of the wave? (c) Given the speed and wavelength of the wave, how could you determine the frequency of the wave? (d) Suggest an independent experiment to determine the frequency of the wave. [Section 6.1]

6.2 A popular kitchen appliance produces electromagnetic radiation with a frequency of 2450 MHz. With reference to Figure 6.4, answer the following: (a) Estimate the wavelength of this radiation. (b) Would the radiation produced by the appliance be visible to the human eye? (c) If the radiation is not visible, do photons of this radiation have more or less energy than photons of visible light? (d) Propose the identity of the kitchen appliance. [Section 6.1]
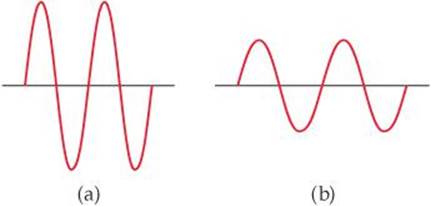
6.3 The following diagrams represent two electromagnetic waves. Which wave corresponds to the higher-energy radiation? Explain. [Section 6.2]
6.4 As shown in the accompanying photograph, an electric stove burner on its highest setting exhibits an orange glow. (a) When the burner setting is changed to low, the burner continues to produce heat but the orange glow disappears. How can this observation be explained with reference to one of the fundamental observations that led to the notion of quanta? (b) Suppose that the energy provided to the burner could be increased beyond the highest setting of the stove. What would we expect to observe with regard to visible light emitted by the burner? [Section 6.2]

6.5 The familiar phenomenon of a rainbow results from the diffraction of sunlight through raindrops. (a) Does the wavelength of light increase or decrease as we proceed outward from the innermost band of the rainbow? (b) Does the frequency of light increase or decrease as we proceed outward? (c) Suppose that instead of sunlight, the visible light from a hydrogen discharge tube (Figure 6.10) was used as the light source. What do you think the resulting “hydrogen discharge rainbow” would look like? [Section 6.3]

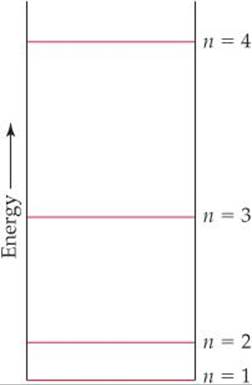
6.6 A certain quantum mechanical system has the energy levels shown in the diagram below. The energy levels are indexed by a single quantum number n that is an integer. (a) As drawn, which quantum numbers are involved in the transition that requires the most energy? (b) Which quantum numbers are involved in the transition that requires the least energy? (c) Based on the drawing, put the following in order of increasing wavelength of the light absorbed or emitted during the transition: (i) n = 1 to n = 2; (ii) n = 3 to n = 2; (iii) n = 2 to n = 4; (iv) n = 3 to n = 1. [Section 6.3]
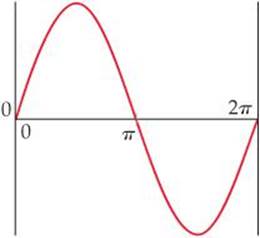
6.7 Consider a fictitious one-dimensional system with one electron. The wave function for the electron, drawn at the top of the next page, is Ψ(x) = sin x from x = 0 to x = 2π. (a) Sketch the probability density, Ψ2(x), from x = 0 to x = 2π. (b) At what value or values of x will there be the greatest probability of finding the electron? (c) What is the probability that the electron will be found at x = π? What is such a point in a wave function called? [Section 6.5]
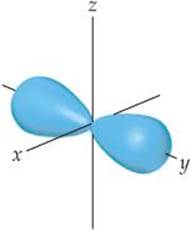
6.8 The contour representation of one of the orbitals for the n = 3 shell of a hydrogen atom is shown below. (a) What is the quantum number l for this orbital? (b) How do we label this orbital? (c) How would you modify this sketch to show the analogous orbital for the n = 4 shell? [Section 6.6]
6.9 The drawing below shows part of the orbital diagram for an element. (a) As drawn, the drawing is incorrect. Why? (b) How would you correct the drawing without changing the number of electrons? (c) To which group in the periodic table does the element belong? [Section 6.8]
![]()
6.10 State where in the periodic table these elements appear:
(a) elements with the valence-shell electron configuration ns2np5
(b) elements that have three unpaired p electrons
(c) an element whose valence electrons are 4s2 4p1
(d) the d-block elements
THE WAVE NATURE OF LIGHT (section 6.1)
6.11 What are the basic SI units for (a) the wavelength of light, (b) the frequency of light, (c) the speed of light?
6.12 (a) What is the relationship between the wavelength and the frequency of radiant energy? (b) Ozone in the upper atmosphere absorbs energy in the 210–230-nm range of the spectrum. In what region of the electromagnetic spectrum does this radiation occur?
______
6.13 Label each of the following statements as true or false. For those that are false, correct the statement. (a) Visible light is a form of electromagnetic radiation. (b) Ultraviolet light has longer wavelengths than visible light. (c) X-rays travel faster than microwaves. (d) Electromagnetic radiation and sound waves travel at the same speed.
6.14 Determine which of the following statements are false and correct them. (a) The frequency of radiation increases as the wavelength increases. (b) Electromagnetic radiation travels through a vacuum at a constant speed, regardless of wavelength. (c) Infrared light has higher frequencies than visible light. (d) The glow from a fireplace, the energy within a microwave oven, and a foghorn blast are all forms of electromagnetic radiation.
______
6.15 Arrange the following kinds of electromagnetic radiation in order of increasing wavelength: infrared, green light, red light, radio waves, X-rays, ultraviolet light.
6.16 List the following types of electromagnetic radiation in order of increasing wavelength: (a) the gamma rays produced by a radioactive nuclide used in medical imaging; (b) radiation from an FM radio station at 93.1 MHz on the dial; (c) a radio signal from an AM radio station at 680 kHz on the dial; (d) the yellow light from sodium vapor streetlights; (e) the red light of a light-emitting diode, such as in a calculator display.
______
6.17 (a) What is the frequency of radiation that has a wavelength of 10 μm, about the size of a bacterium? (b) What is the wavelength of radiation that has a frequency of 5.50 × 1014 s–1? (c) Would the radiations in part (a) or part (b) be visible to the human eye? (d) What distance does electromagnetic radiation travel in 50.0 μs?
6.18 (a) What is the frequency of radiation whose wavelength is 5.0 × 10–5 m? (b) What is the wavelength of radiation that has a frequency of 2.5 × 108 s–1? (c) Would the radiations in part (a) or part (b) be detected by an X-ray detector? (d) What distance does electromagnetic radiation travel in 10.5 fs?
______
6.19 An argon ion laser emits light at 532 nm. What is the frequency of this radiation? Using Figure 6.4, predict the color associated with this wavelength.
6.20 It is possible to convert radiant energy into electrical energy using photovoltaic cells. Assuming equal efficiency of conversion, would infrared or ultraviolet radiation yield more electrical energy on a per-photon basis?
QUANTIZED ENERGY AND PHOTONS (section 6.2)
6.21 If human height were quantized in one-foot increments, what would happen to the height of a child as she grows up?
6.22 Einstein's 1905 paper on the photoelectric effect was the first important application of Planck's quantum hypothesis. Describe Planck's original hypothesis, and explain how Einstein made use of it in his theory of the photoelectric effect.
______
6.23 (a) Calculate the energy of a photon of electromagnetic radiation whose frequency is 6.75 × 1012 s–1. (b) Calculate the energy of a photon of radiation whose wavelength is 322 nm. (c) What wavelength of radiation has photons of energy 2.87 × 10–18 J?
6.24 (a) A red laser pointer emits light with a wavelength of 650 nm. What is the frequency of this light? (b) What is the energy of one of these photons? (c) The laser pointer emits light because electrons in the material are excited (by a battery) from their ground state to an upper excited state. When the electrons return to the ground state, they lose the excess energy in the form of 650 nm photons. What is the energy gap between the ground state and excited state in the laser material?
______
6.25 (a) Calculate and compare the energy of a photon of wavelength 3.3 μm with that of wavelength 0.154 nm. (b) Use Figure 6.4 to identify the region of the electromagnetic spectrum to which each belongs.
6.26 An AM radio station broadcasts at 1010 kHz, and its FM partner broadcasts at 98.3 MHz. Calculate and compare the energy of the photons emitted by these two radio stations.
______
6.27 One type of sunburn occurs on exposure to UV light of wavelength in the vicinity of 325 nm. (a) What is the energy of a photon of this wavelength? (b) What is the energy of a mole of these photons? (c) How many photons are in a 1.00 mJ burst of this radiation? (d) These UV photons can break chemical bonds in your skin to cause sunburn—a form of radiation damage. If the 325-nm radiation provides exactly the energy to break an average chemical bond in the skin, estimate the average energy of these bonds in kJ/mol.
6.28 The energy from radiation can be used to cause the rupture of chemical bonds. A minimum energy of 941 kJ/molis required to break the nitrogen–nitrogen bond in N2. What is the longest wavelength of radiation that possesses the necessary energy to break the bond? What type of electromagnetic radiation is this?
______
6.29 A diode laser emits at a wavelength of 987 nm. (a) In what portion of the electromagnetic spectrum is this radiation found? (b) All of its output energy is absorbed in a detector that measures a total energy of 0.52 J over a period of 32 s. How many photons per second are being emitted by the laser?
6.30 A stellar object is emitting radiation at 3.55 mm. (a) What type of electromagnetic spectrum is this radiation? (b) If a detector is capturing 3.2 × 108 photons per second at this wavelength, what is the total energy of the photons detected in one hour?
______
6.31 Molybdenum metal must absorb radiation with a minimum frequency of 1.09 × 1015 s–1 before it can eject an electron from its surface via the photoelectric effect. (a) What is the minimum energy needed to eject an electron? (b) What wavelength of radiation will provide a photon of this energy? (c) If molybdenum is irradiated with light of wavelength of 120 nm, what is the maximum possible kinetic energy of the emitted electrons?
6.32 Sodium metal requires a photon with a minimum energy of 4.41 × 10–19 J to emit electrons. (a) What is the minimum frequency of light necessary to emit electrons from sodium via the photoelectric effect? (b) What is the wavelength of this light? (c) If sodium is irradiated with light of 405 nm, what is the maximum possible kinetic energy of the emitted electrons? (d) What is the maximum number of electrons that can be freed by a burst of light whose total energy is 1.00 μJ?
BOHR'S MODEL; MATTER WAVES (sections 6.3 and 6.4)
6.33 Explain how the existence of line spectra is consistent with Bohr's theory of quantized energies for the electron in the hydrogen atom.
6.34 (a) In terms of the Bohr theory of the hydrogen atom, what process is occurring when excited hydrogen atoms emit radiant energy of certain wavelengths and only those wavelengths? (b) Does a hydrogen atom “expand” or “contract” as it moves from its ground state to an excited state?
______
6.35 Is energy emitted or absorbed when the following electronic transitions occur in hydrogen: (a) from n = 4 to n = 2, (b) from an orbit of radius 2.12 Å to one of radius 8.46 Å, (c) an electron adds to the H+ ion and ends up in the n = 3 shell?
6.36 Indicate whether energy is emitted or absorbed when the following electronic transitions occur in hydrogen: (a) from n = 2 to n = 6, (b) from an orbit of radius 4.76 Å to one of radius 0.529 Å, (c) from the n = 6 to the n = 9 state.
______
6.37 (a) Using Equation 6.5, calculate the energy of an electron in the hydrogen atom when n = 2 and when n = 6. Calculate the wavelength of the radiation released when an electron moves from n = 6 to n = 2. (b) Is this line in the visible region of the electromagnetic spectrum? If so, what color is it?
6.38 (a) Calculate the energies of an electron in the hydrogen atom for n = 1 and for n = ∞. How much energy does it require to move the electron out of the atom completely (from n = 1 to n = ∞), according to Bohr? Put your answer in kJ/mol. (b) The energy for the process H + energy → H+ + e– is called the ionization energy of hydrogen. The experimentally determined value for the ionization energy of hydrogen is 1310 kJ/mol. How does this compare to your calculation?
______
6.39 The visible emission lines observed by Balmer all involved nf = 2. (a) Explain why only the lines with nf = 2 were observed in the visible region of the electromagnetic spectrum. (b) Calculate the wavelengths of the first three lines in the Balmer series—those for which ni = 3, 4, and 5—and identify these lines in the emission spectrum shown in Figure 6.11.
6.40 The Lyman series of emission lines of the hydrogen atom are those for which nf = 1. (a) Determine the region of the electromagnetic spectrum in which the lines of the Lyman series are observed. (b) Calculate the wavelengths of the first three lines in the Lyman series—those for which ni = 2, 3, and 4.
______
6.41 One of the emission lines of the hydrogen atom has a wavelength of 93.8 nm. (a) In what region of the electromagnetic spectrum is this emission found? (b) Determine the initial and final values of n associated with this emission.
6.42 The hydrogen atom can absorb light of wavelength 2626 nm. (a) In what region of the electromagnetic spectrum is this absorption found? (b) Determine the initial and final values of n associated with this absorption.
______
6.43 Use the de Broglie relationship to determine the wavelengths of the following objects: (a) an 85-kg person skiing at 50 km/hr, (b) a 10.0-g bullet fired at 250 m/s, (c) a lithium atom moving at 2.5 × 105 m/s, (d) an ozone (O3) molecule in the upper atmosphere moving at 550 m/s.
6.44 Among the elementary subatomic particles of physics is the muon, which decays within a few nanoseconds after formation. The muon has a rest mass 206.8 times that of an electron. Calculate the de Broglie wavelength associated with a muon traveling at a velocity of 8.85 × 105cm/s.
______
6.45 Neutron diffraction is an important technique for determining the structures of molecules. Calculate the velocity of a neutron needed to achieve a wavelength of 0.955 Å. (Refer to the inside cover for the mass of the neutron).
6.46 The electron microscope has been widely used to obtain highly magnified images of biological and other types of materials. When an electron is accelerated through a particular potential field, it attains a speed of 8.95 × 106 m/s. What is the characteristic wavelength of this electron? Is the wavelength comparable to the size of atoms?
______
6.47 Using Heisenberg's uncertainty principle, calculate the uncertainty in the position of (a) a 1.50-mg mosquito moving at a speed of 1.40 m/s if the speed is known to within ± 0.01 m/s; (b) a proton moving at a speed of (5.00±0.01) × 104 m/s. (The mass of a proton is given in the table of fundamental constants in the inside cover of the text.)
6.48 Calculate the uncertainty in the position of (a) an electron moving at a speed of (3.00±0.01) × 105 m/s, (b) a neutron moving at this same speed. (The masses of an electron and a neutron are given in the table of fundamental constants in the inside cover of the text.) (c) What are the implications of these calculations to our model of the atom?
QUANTUM MECHANICS AND ATOMIC ORBITALS (sections 6.5 and 6.6)
6.49 (a) Why does the Bohr model of the hydrogen atom violate the uncertainty principle? (b) In what way is the description of the electron using a wave function consistent with de Broglie's hypothesis? (c) What is meant by the term probability density? Given the wave function, how do we find the probability density at a certain point in space?
6.50 (a) According to the Bohr model, an electron in the ground state of a hydrogen atom orbits the nucleus at a specific radius of 0.53 Å. In the quantum mechanical description of the hydrogen atom, the most probable distance of the electron from the nucleus is 0.53 Å. Why are these two statements different? (b) Why is the use of Schrödinger's wave equation to describe the location of a particle very different from the description obtained from classical physics? (c) In the quantum mechanical description of an electron, what is the physical significance of the square of the wave function, Ψ2?
______
6.51 (a) For n = 4, what are the possible values of l? (b) For l = 2, what are the possible values of ml? (c) If ml is 2, what are the possible values for l?
6.52 How many possible values for l and ml are there when (a) n = 3; (b) n = 5?
______
6.53 Give the numerical values of n and l corresponding to each of the following orbital designations: (a) 3p, (b) 2s, (c) 4f, (d) 5d.
6.54 Give the values for n, l, and ml for (a) each orbital in the 2p subshell, (b) each orbital in the 5d subshell.
______
6.55 Which of the following represent impossible combinations of n and l: (a) 1p, (b) 4s, (c) 5f, (d) 2d?
6.56 For the table that follows, write which orbital goes with the quantum numbers. Don't worry about x, y, z subscripts. If the quantum numbers are not allowed, write “not allowed.”
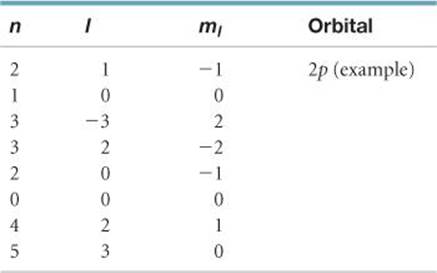
______
6.57 Sketch the shape and orientation of the following types of orbitals: (a) s, (b) pz, (c) dxy.
6.58 Sketch the shape and orientation of the following types of orbitals: (a) px, (b) dz2, (c) dx2-y2.
______
6.59 (a) What are the similarities and differences between the 1s and 2s orbitals of the hydrogen atom? (b) In what sense does a 2p orbital have directional character? Compare the “directional” characteristics of the px and dx2-y2 orbitals. (That is, in what direction or region of space is the electron density concentrated?) (c) What can you say about the average distance from the nucleus of an electron in a 2s orbital as compared with a 3s orbital? (d) For the hydrogen atom, list the following orbitals in order of increasing energy (that is, most stable ones first): 4f, 6s, 3d, 1s, 2p.
6.60 (a) With reference to Figure 6.18, what is the relationship between the number of nodes in an s orbital and the value of the principal quantum number? (b) Identify the number of nodes; that is, identify places where the electron density is zero, in the 2px orbital; in the 3s orbital. (c)What information is obtained from the radial probability functions in Figure 6.18? (d) For the hydrogen atom, list the following orbitals in order of increasing energy: 3s, 2s, 2p, 5s, 4d.
MANY-ELECTRON ATOMS AND ELECTRON CONFIGURATIONS (sections 6.7–6.9)
6.61 For a given value of the principal quantum number, n, how do the energies of the s, p, d, and f subshells vary for (a) hydrogen, (b) a many-electron atom?
6.62 (a) The average distance from the nucleus of a 3s electron in a chlorine atom is smaller than that for a 3p electron. In light of this fact, which orbital is higher in energy? (b) Would you expect it to require more or less energy to remove a 3s electron from the chlorine atom, as compared with a 2p electron? Explain.
______
6.63 (a) What experimental evidence is there for the electron having a “spin”? (b) Draw an energy-level diagram that shows the relative energetic positions of a 1s orbital and a 2s orbital. Put two electrons in the 1s orbital. (c) Draw an arrow showing the excitation of an electron from the 1s to the 2s orbital.
6.64 (a) State the Pauli exclusion principle in your own words. (b) The Pauli exclusion principle is, in an important sense, the key to understanding the periodic table. Explain.
______
6.65 What is the maximum number of electrons that can occupy each of the following subshells: (a) 3p, (b) 5d, (c) 2s, (d) 4f?
6.66 What is the maximum number of electrons in an atom that can have the following quantum numbers: (a) n = 2, ![]() (b) n = 5, l = 3; (c) n = 4, l = 3, ml = −3; (d) n = 4,l = 0,ml = 0?
(b) n = 5, l = 3; (c) n = 4, l = 3, ml = −3; (d) n = 4,l = 0,ml = 0?
______
6.67 (a) What are “valence electrons”? (b) What are “core electrons”? (c) What does each box in an orbital diagram represent? (d) What quantity is represented by the half arrows in an orbital diagram?
6.68 For each element, indicate the number of valence electrons, core electrons, and unpaired electrons in the ground state: (a) carbon, (b) phosphorus, (c) neon.
______
6.69 Write the condensed electron configurations for the following atoms, using the appropriate noble-gas core abbreviations: (a) Cs, (b) Ni, (c) Se, (d) Cd, (e) U, (f) Pb.
6.70 Write the condensed electron configurations for the following atoms and indicate how many unpaired electrons each has: (a) Mg, (b) Ge, (c) Br, (d) V, (e) Y, (f) Lu.
______
6.71 Identify the specific element that corresponds to each of the following electron configurations and indicate the number of unpaired electrons for each: (a) 1s22s2, (b) 1s22s22p4, (c) [Ar]4s13d5, (d) [Kr]5s24d105p4.
6.72 Identify the group of elements that corresponds to each of the following generalized electron configurations and indicate the number of unpaired electrons for each:
(a) [noble gas] ns2np5
(b) [noble gas] ns2(n – 1)d2
(c) [noble gas] ns2(n – 1)d10np1
(d) [noble gas] ns2(n – 2)f 6
______
6.73 What is wrong with the following electron configurations for atoms in their ground states? (a) ls22s23s1, (b) [Ne]2s22p3, (c) [Ne]3s23d5.
6.74 The following electron configurations represent excited states. Identify the element, and write its ground-state condensed electron configuration. (a) 1s22s23p24p1, (b) [Ar]3d104s14p45s1, (c) [Kr]4d65s25p1.
ADDITIONAL EXERCISES
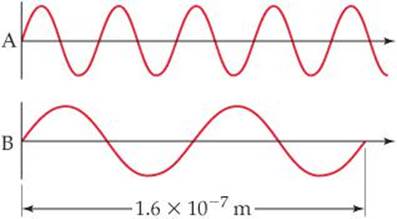
6.75 Consider the two waves shown here, which we will consider to represent two electromagnetic radiations:
(a) What is the wavelength of wave A? Of wave B?
(b) What is the frequency of wave A? Of wave B?
(c) Identify the regions of the electromagnetic spectrum to which waves A and B belong.
6.76 If you put 120 volts of electricity through a pickle, the pickle will smoke and start glowing orange-yellow. The light is emitted because sodium ions in the pickle become excited; their return to the ground state results in light emission. (a) The wavelength of this emitted light is 589 nm. Calculate its frequency. (b) What is the energy of 0.10 mole of these photons? (c) Calculate the energy gap between the excited and ground states for the sodium ion. (d) If you soaked the pickle for a long time in a different salt solution, such as strontium chloride, would you still observe 589-nm light emission? Why or why not?
6.77 Certain elements emit light of a specific wavelength when they are burned. Historically, chemists used such emission wavelengths to determine whether specific elements were present in a sample. Characteristic wavelengths for some of the elements are given in the following table:
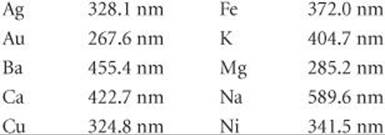
(a) Determine which elements emit radiation in the visible part of the spectrum. (b) Which element emits photons of highest energy? Of lowest energy? (c) When burned, a sample of an unknown substance is found to emit light of frequency 6.59 × 1014 s–1. Which of these elements is probably in the sample?
6.78 In June 2004, the Cassini–Huygens spacecraft began orbiting Saturn and transmitting images to Earth. The closest distance between Saturn and Earth is 746 million miles. What is the minimum amount of time it takes for the transmitted signals to travel from the spacecraft to Earth?
6.79 The rays of the Sun that cause tanning and burning are in the ultraviolet portion of the electromagnetic spectrum. These rays are categorized by wavelength. So-called UV-A radiation has wavelengths in the range of 320–380 nm, whereas UV-B radiation has wavelengths in the range of 290–320 nm. (a) Calculate the frequency of light that has a wavelength of 320 nm. (b) Calculate the energy of a mole of 320-nm photons. (c) Which are more energetic, photons of UV-A radiation or photons of UV-B radiation? (d) The UV-B radiation from the Sun is considered a greater cause of sunburn in humans than is UV-A radiation. Is this observation consistent with your answer to part (c)?
6.80 The watt is the derived SI unit of power, the measure of energy per unit time: 1 W = 1 J-s. A semiconductor laser in a CD player has an output wavelength of 780 nm and a power level of 0.10 mW. How many photons strike the CD surface during the playing of a CD 69 minutes in length?
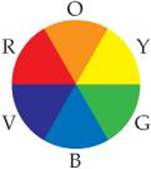
6.81 Carotenoids are yellow, orange, and red pigments synthesized by plants. The observed color of an object is not the color of light it absorbs but rather the complementary color, as described by a color wheel such as the one shown here. On this wheel, complementary colors are across from each other. (a) Based on this wheel, what color is absorbed most strongly if a plant is orange? (b) If a particular carotenoid absorbs photons at 455 nm, what is the energy of the photon?
6.82 A photocell is a device used to measure the intensity of light. In a certain experiment, when light of wavelength 630 nm is directed onto the photocell, electrons are emitted at the rate of 2.6 × 10−12 C/s (coulombs per second). Assume that each photon that impinges on the photocell emits one electron. How many photons per second are striking the photocell? How much energy per second is the photocell absorbing?
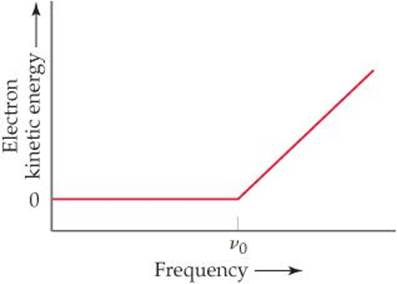
6.83 In an experiment to study the photoelectric effect, a scientist measures the kinetic energy of ejected electrons as a function of the frequency of radiation hitting a metal surface. She obtains the following plot. The point labeled “v0” corresponds to light with a wavelength of 680 nm.(a) What is the value of n0 in s–1? (b) What is the value of the work function of the metal in units of kJ/mol of ejected electrons? (c) What happens when the metal is irradiated with light of frequency less than v0? (d) Note that when the frequency of the light is greater than v0, the plot shows a straight line with a nonzero slope. Why is this the case? (e) Can you determine the slope of the line segment discussed in part (d)? Explain.
6.84 The human retina has three types of receptor cones, each sensitive to a different range of wavelengths of visible light, as shown in this figure (the colors are merely to differentiate the three curves from one another; they do not indicate the actual colors represented by each curve):
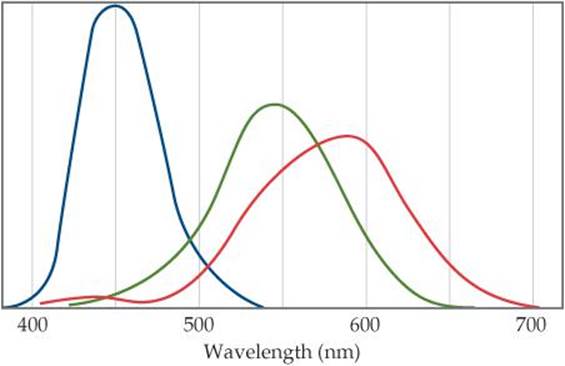
(a) Estimate the energies of photons with wavelengths at the maximum for each type of cone. (b) The color of the sky is due to scattering of solar light by the molecules of the atmosphere. Lord Rayleigh was one of the first to study scattering of this kind. He showed that the amount of scattering for very small particles such as molecules is inversely proportional to the fourth power of the wavelength. Estimate the ratio of the scattering efficiency of light at the wavelength of the maximum for the “blue” cones, as compared with that for the “green” cones. (c) Explain why the sky appears blue even though all wavelengths of solar light are scattered by the atmosphere.
6.85 The series of emission lines of the hydrogen atom for which nf = 3 is called the Paschen series. (a) Determine the region of the electromagnetic spectrum in which the lines of the Paschen series are observed. (b) Calculate the wavelengths of the first three lines in the Paschen series—those for which ni = 4, 5, and 6.
6.86 When the spectrum of light from the Sun is examined in high resolution in an experiment similar to that illustrated in Figure 6.9, dark lines are evident. These are called Fraunhofer lines, after the scientist who studied them extensively in the early nineteenth century. Altogether, about 25,000 lines have been identified in the solar spectrum between 2950 Å and 10,000 Å. The Fraunhofer lines are attributed to absorption of certain wavelengths of the Sun's “white” light by gaseous elements in the Sun's atmosphere. (a) Describe the process that causes absorption of specific wavelengths of light from the solar spectrum. (b) To determine which Fraunhofer lines belonged to a given element, say neon, what experiments could a scientist conduct here on Earth?
[6.87] Bohr's model can be used for hydrogen-like ions—ions that have only one electron, such as He+ and Li2+. (a) Why is the Bohr model applicable to He+ ions but not to neutral He atoms? (b) The ground-state energies of H, He+, and Li2+ are tabulated as follows:

By examining these numbers, propose a relationship between the ground-state energy of hydrogen-like systems and the nuclear charge, Z. (c) Use the relationship you derive in part (b) to predict the ground-state energy of the C5+ ion.
[6.88] An electron is accelerated through an electric potential to a kinetic energy of 18.6 keV. What is its characteristic wavelength? [Hint: Recall that the kinetic energy of a moving object is ![]() , where m is the mass of the object and v is the speed of the object.]
, where m is the mass of the object and v is the speed of the object.]
6.89 In the television series star Trek, the transporter beam is a device used to “beam down” people from the starship Enterprise to another location, such as the surface of a planet. The writers of the show put a “Heisenberg compensator” into the transporter beam mechanism. Explain why such a compensator (which is entirely fictional) would be necessary to get around Heisenberg's uncertainty principle.
6.90 Which of the quantum numbers governs (a) the shape of an orbital, (b) the energy of an orbital, (c) the spin properties of the electron, (d) the spatial orientation of the orbital?
[6.91] Consider the discussion of radial probability functions in “A Closer Look” in Section 6.6. (a) What is the difference between the probability density as a function of r and the radial probability function as a function of r? (b) What is the significance of the term 4πr2 in the radial probability functions for the s orbitals? (c) Based on Figures 6.18 and 6.21, make sketches of what you think the probability density as a function of r and the radial probability function would look like for the 4s orbital of the hydrogen atom.
[6.92] For orbitals that are symmetric but not spherical, the contour representations (as in Figures 6.22 and 6.23) suggest where nodal planes exist (that is, where the electron density is zero). For example, the px orbital has a node wherever x = 0. This equation is satisfied by all points on the yz plane, so this plane is called a nodal plane of the px orbital. (a) Determine the nodal plane of the pz orbital. (b) What are the two nodal planes of the dxy orbital? (c) What are the two nodal planes of the dx2-y2 orbital?
[6.93] The “Chemistry and Life” box in Section 6.7 described the techniques called NMR and MRI. (a) Instruments for obtaining MRI data are typically labeled with a frequency, such as 600 MHz. Why do you suppose this label is relevant to the experiment? (b) What is the value of ΔEin Figure 6.27 that would correspond to the absorption of a photon of radiation with frequency 450 MHz? (c) In general, the stronger the magnetic field, the greater the information obtained from an NMR or MRI experiment. Why do you suppose this is the case?
6.94 Suppose that the spin quantum number, ms, could have three allowed values instead of two. How would this affect the number of elements in the first four rows of the periodic table?
6.95 Using the periodic table as a guide, write the condensed electron configuration and determine the number of unpaired electrons for the ground state of (a) Si, (b) Zn, (c) Zr, (d) Sn, (e) Ba, (f) Tl.
6.96 Scientists have speculated that element 126 might have a moderate stability, allowing it to be synthesized and characterized. Predict what the condensed electron configuration of this element might be.
INTEGRATIVE EXERCISES
6.97 Microwave ovens use microwave radiation to heat food. The energy of the microwaves is absorbed by water molecules in food and then transferred to other components of the food. (a) Suppose that the microwave radiation has a wavelength of 11.2 cm. How many photons are required to heat 200 mL of coffee from 23 °C to 60 °C? (b) Suppose the microwave's power is 900 W (1 Watt = 1 joule-second). How long would you have to heat the coffee in part (a)?
6.98 The stratospheric ozone (O3) layer helps to protect us from harmful ultraviolet radiation. It does so by absorbing ultraviolet light and falling apart into an O2 molecule and an oxygen atom, a process known as photodissociation.
O3(g) → O2(g) + O(g)
Use the data in Appendix C to calculate the enthalpy change for this reaction. What is the maximum wavelength a photon can have if it is to possess sufficient energy to cause this dissociation? In what portion of the spectrum does this wavelength occur?
6.99 The discovery of hafnium, element number 72, provided a controversial episode in chemistry. G. Urbain, a French chemist, claimed in 1911 to have isolated an element number 72 from a sample of rare earth (elements 58-71) compounds. However, Niels Bohr believed that hafnium was more likely to be found along with zirconium than with the rare earths. D. Coster and G. von Hevesy, working in Bohr's laboratory in Copenhagen, showed in 1922 that element 72 was present in a sample of Norwegian zircon, an ore of zirconium. (The name hafnium comes from the Latin name for Copenhagen, Hafnia). (a) How would you use electron configuration arguments to justify Bohr's prediction? (b) Zirconium, hafnium's neighbor in group 4B, can be produced as a metal by reduction of solid ZrCl4 with molten sodium metal. Write a balanced chemical equation for the reaction. Is this an oxidation-reduction reaction? If yes, what is reduced and what is oxidized? (c) Solid zirconium dioxide, ZrO2, is reacted with chlorine gas in the presence of carbon. The products of the reaction are ZrCl4 and two gases, CO2and CO in the ratio 1:2. Write a balanced chemical equation for the reaction. Starting with a 55.4-g sample of ZrO2, calculate the mass of ZrCl4 formed, assuming that ZrO2 is the limiting reagent and assuming 100% yield. (d) Using their electron configurations, account for the fact that Zr and Hf form chlorides MCl4 and oxides MO2.
6.100 (a) Account for formation of the following series of oxides in terms of the electron configurations of the elements and the discussion of ionic compounds in Section 2.7: K2O, CaO, Sc2O3, TiO2, V2O5, CrO3. (b) Name these oxides. (c) Consider the metal oxides whose enthalpies of formation (in kJ mol-1) are listed here.
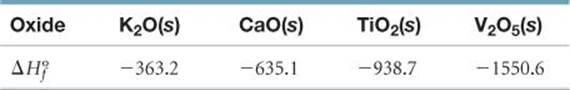
Calculate the enthalpy changes in the following general reaction for each case:
MnOm(s) + H2(g) → nM(s) + mH2O(g)
(You will need to write the balanced equation for each case and then compute ΔHo.) (d) Based on the data given, estimate a value of ![]() for Sc2O3(s).
for Sc2O3(s).
6.101 The first 25 years of the twentieth century were momentous for the rapid pace of change in scientists' understanding of the nature of matter. (a) How did Rutherford's experiments on the scattering of a particles by a gold foil set the stage for Bohr's theory of the hydrogen atom?(b) In what ways is de Broglie's hypothesis, as it applies to electrons, consistent with J. J. Thomson's conclusion that the electron has mass? In what sense is it consistent with proposals preceding Thomson's work that the cathode rays are a wave phenomenon?
[6.102] The two most common isotopes of uranium are 235U and 238U. (a) Compare the number of protons, the number of electrons, and the number of neutrons in atoms of these two isotopes. (b) Using the periodic table in the front inside cover, write the electron configuration for a U atom. (c) Compare your answer to part (b) to the electron configuration given in Figure 6.31. How can you explain any differences between these two electron configurations? (d) 238U undergoes radioactive decay to 234Th. How many protons, electrons, and neutrons are gained or lost by the 238U atom during this process? (e) Examine the electron configuration for Th in Figure 6.31. Are you surprised by what you find? Explain.
6.103 Imagine sunlight falling on three square areas. One is an inert black material. The second is a photovoltaic cell surface, which converts radiant energy into electricity. The third is an area on a green tree leaf. Draw diagrams that show the energy conversions in each case, usingFigure 5.9 as a model. How are these three examples related to the idea of sustainable energy sources?