CHEMISTRY THE CENTRAL SCIENCE
7 PERIODIC PROPERTIES OF THE ELEMENTS
EXERCISES
VISUALIZING CONCEPTS
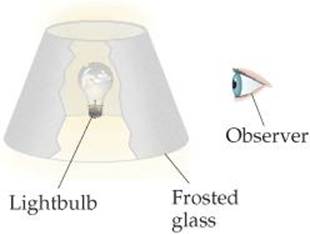
7.1 We can draw an analogy between the attraction of an electron to a nucleus and seeing a lightbulb—in essence, the more nuclear charge the electron “sees,” the greater the attraction. (a) Within this analogy, discuss how the screening by core electrons is analogous to putting a frosted-glass lampshade between the lightbulb and your eyes, as shown in the illustration. (b) Explain how we could mimic moving to the right in a row of the periodic table by changing the wattage of the lightbulb. (c) How would you change the wattage of the bulb and/or the frosted glass to mimic the effect of moving down a column of the periodic table? [Section 7.2]
7.2 If you look up the radius of the sulfur atom in this book, you will find just one number: 1.02 Å. However, if you look deeper into the chemical literature, you can find another number for the radius of a sulfur atom: the nonbonding radius of 1.80 Å. This is a very large difference! Explain. [Section 7.3]
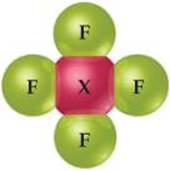
7.3 Consider the A2X4 molecule depicted here, where A and X are elements. The A—A bond length in this molecule is d1, and the four A—X bond lengths are each d2. (a) In terms of d1 and d2, how could you define the bonding atomic radii of atoms A and X? (b) In terms of d1 and d2, what would you predict for the X—X bond length of an X2 molecule? [Section 7.3]
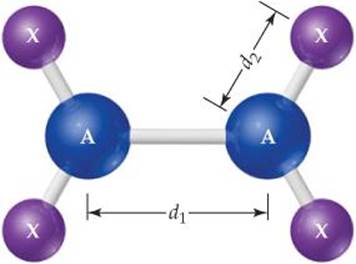
7.4 Make a simple sketch of the shape of the main part of the periodic table, as shown. (a) Ignoring H and He, write a single straight arrow from the element with the smallest bonding atomic radius to the element with the largest. (b) Ignoring H and He, write a single straight arrow from the element with the smallest first ionization energy to the element with the largest. (c) What significant observation can you make from the arrows you drew in parts (a) and (b)? [Sections 7.3 and 7.4]
7.5 In the chemical process called electron transfer, an electron is transferred from one atom or molecule to another. (We will talk about electron transfer extensively in Chapter 20.) A simple electron transfer reaction is
![]()
In terms of the ionization energy and electron affinity of atom A, what is the energy change for this reaction? For a representative nonmetal such as chlorine, is this process exothermic? For a representative metal such as sodium, is this process exothermic? [Sections 7.4 and 7.5]
7.6 An element X reacts with F2(g) to form the molecular product shown here. (a) Write a balanced equation for this reaction (do not worry about the phases for X and the product). (b) Do you think that X is a metal or nonmetal? Explain. [Section 7.6]

PERIODIC TABLE; EFFECTIVE NUCLEAR CHARGE (sections 7.1 and 7.2)
7.7 Explain the structure of the periodic table—two columns on the left, a block of ten for the transition metals, a block of six on the right, and a pair of 14-member rows below, with reference to the orbitals we discussed in Chapter 6.
7.8 The prefix eka- comes from the Sanskrit word for “one.” Mendeleev used this prefix to indicate that the unknown element was one place away from the known element that followed the prefix. For example, eka-silicon, which we now call germanium, is one element below silicon. Mendeleev also predicted the existence of eka-manganese, which was not experimentally confirmed until 1937 because this element is radioactive and does not occur in nature. Based on the periodic table shown in Figure 7.1, what do we now call the element Mendeleev called eka-manganese?
______
7.9 You might have expected that the elements would have been discovered in order of their relative abundance in the Earth's crust (Figure 1.6), but this is not the case. Suggest a general reason.
7.10 (a) Moseley's experiments on X-rays emitted from atoms led to the concept of atomic numbers. Where exactly do these X-rays come from? Draw an energy-level diagram to explain. (b) Why are chemical and physical properties of the elements more closely related to atomic number than they are to atomic weight?
______
7.11 (a) What is meant by the term effective nuclear charge? (b) How does the effective nuclear charge experienced by the valence electrons of an atom vary going from left to right across a period of the periodic table?
7.12 (a) How is the concept of effective nuclear charge used to simplify the numerous electron–electron repulsions in a many-electron atom? (b) Which experiences a greater effective nuclear charge in a Be atom, the 1s electrons or the 2s electrons? Explain.
______
7.13 Detailed calculations show that the value of Zeff for the outermost electrons in Na and K atoms is 2.51+ and 3.49+, respectively. (a) What value do you estimate for Zeff experienced by the outermost electron in both Na and K by assuming core electrons contribute 1.00 and valence electrons contribute 0.00 to the screening constant? (b) What values do you estimate for Zeff using Slater's rules? (c) Which approach gives a more accurate estimate of Zeff? (d) Does either method of approximation account for the gradual increase in Zeff that occurs upon moving down a group? (e) Predict Zeff for the outermost electrons in the Rb atom based on the calculations for Na and K.
7.14 Detailed calculations show that the value of Zeff for the outermost electrons in Si and Cl atoms is 4.29+ and 6.12+, respectively. (a) What value do you estimate for Zeff experienced by the outermost electron in both Si and Cl by assuming core electrons contribute 1.00 and valence electrons contribute 0.00 to the screening constant? (b) What values do you estimate for Zeff using Slater's rules? (c) Which approach gives a more accurate estimate of Zeff? (d) Which method of approximation more accurately accounts for the steady increase in Zeff that occurs upon moving left to right across a period? (e) Predict Zeff for a valence electron in P, phosphorus, based on the calculations for Si and Cl.
______
7.15 Which will experience the greater effective nuclear charge, the electrons in the n = 3 shell in Ar or the n = 3 shell in Kr? Which will be closer to the nucleus? Explain.
7.16 Arrange the following atoms in order of increasing effective nuclear charge experienced by the electrons in the n = 3 electron shell: K, Mg, P, Rh, and Ti. Explain the basis for your order.
ATOMIC AND IONIC RADII (section 7.3)
7.17 (a) Because an exact outer boundary cannot be measured or even calculated for an atom, how are atomic radii determined? (b) What is the difference between a bonding radius and a nonbonding radius? (c) For a given element, which one is larger? (d) If a free atom reacts to become part of a molecule, would you say that the atom gets smaller or larger?
7.18 (a) Why does the quantum mechanical description of many-electron atoms make it difficult to define a precise atomic radius? (b) When nonbonded atoms come up against one another, what determines how closely the nuclear centers can approach?
______
7.19 Tungsten has the highest melting point of any metal in the periodic table: 3422 °C. The distance between W atoms in tungsten metal is 2.74 Å. (a) What is the atomic radius of a tungsten atom in this environment? (This radius is called the metallic radius.) (b) If you put tungsten metal under high pressure, predict what would happen to the distance between W atoms.
7.20 Based on the radii presented in Figure 7.6, predict the distance between Si atoms in solid silicon. How does this compare to the distance between the C atoms in diamond, which has the same structure as solid silicon?
______
7.21 Estimate the As—I bond length from the data in Figure 7.6, and compare your value to the experimental As∈I bond length in arsenic triiodide, AsI3, 2.55 Å.
7.22 The experimental Bi—I bond length in bismuth triiodide, BiI3, is 2.81 Å. Based on this value and data in Figure 7.6, predict the atomic radius of Bi.
______
7.23 How do the sizes of atoms change as we move (a) from left to right across a row in the periodic table, (b) from top to bottom in a group in the periodic table? (c) Arrange the following atoms in order of increasing atomic radius: O, Si, I, Ge.
7.24 (a) Among the nonmetallic elements, the change in atomic radius in moving one place left or right in a row is smaller than the change in moving one row up or down. Explain these observations. (b) Arrange the following atoms in order of increasing atomic radius: Si, Al, Ge, Ga.
______
7.25 Using only the periodic table, arrange each set of atoms in order from largest to smallest: (a) K, Li, Cs; (b) Pb, Sn, Si; (c) F, O, N.
7.26 Using only the periodic table, arrange each set of atoms in order of increasing radius: (a) Ba, Ca, Na; (b) Sn, Sb, As; (c) Al, Be, Si.
______
7.27 True or False: (a) Cations are larger than their corresponding neutral atoms. (b) Li+ is smaller than Li. (c) Cl− is bigger than I−.
7.28 Explain the following variations in atomic or ionic radii:
(a) I− > I > I+,
(b) Ca2+ 7 Mg2+ 7 Be2+,
(c) Fe > Fe2+ > Fe3+.
______
7.29 In the reaction
which sphere represents a metal and which represents a non-metal? Explain your answer.
7.30 Which of these spheres represents F, which represents Br, and which represents Br–?

______
7.31 (a) What is an isoelectronic series? (b) Which neutral atom is isoelectronic with each of the following ions: Ga3+, Zr4+, Mn7+, I−, Pb2+?
7.32 Identify at least two ions that have the following ground-state electron configurations: (a) [Ar]; (b) [Ar]3d5; (c) [Kr]5s24d10.
______
7.33 Some ions do not have a corresponding neutral atom that has the same electron configuration. For each of the following ions, identify the neutral atom that has the same number of electrons and determine if this atom has the same electron configuration. If such an atom does not exist, explain why.
(a) Cl−,
(b) Sc3+,
(c) Fe2+,
(d) Zn2+,
(e) Sn4+.
7.34 Consider the isoelectronic ions F− and Na+. (a) Which ion is smaller? (b) Using Equation 7.1 and assuming that core electrons contribute 1.00 and valence electrons contribute 0.00 to the screening constant, S, calculate Zeff for the 2p electrons in both ions. (c) Repeat this calculation using Slater's rules to estimate the screening constant, S. (d) For isoelectronic ions, how are effective nuclear charge and ionic radius related?
______
7.35 Consider the isoelectronic ions Cl− and K+. (a) Which ion is smaller? (b) Using Equation 7.1 and assuming that core electrons contribute 1.00 and valence electrons contribute nothing to the screening constant, S, calculate Zeff for these two ions. (c) Repeat this calculation using Slater's rules to estimate the screening constant, S. (d) For isoelectronic ions, how are effective nuclear charge and ionic radius related?
7.36 Consider S, Cl, and K and their most common ions. (a) List the atoms in order of increasing size. (b) List the ions in order of increasing size. (c) Explain any differences in the orders of the atomic and ionic sizes.
______
7.37 For each of the following sets of atoms and ions, arrange the members in order of increasing size: (a) Se2−, Te2−, Se; (b) Co3+, Fe2+, Fe3+; (c) Ca, Ti4+, Sc3+; (d) Be2+, Na+, Ne.
7.38 In the ionic compounds LiF, NaCl, KBr, and RbI, the measured cation–anion distances are 2.01 Å (Li–F), 2.82 Å (Na–Cl), 3.30 Å (K–Br), and 3.67 Å (Rb–I), respectively. (a) Predict the cation–anion distance using the values of ionic radii given in Figure 7.7. (b) Calculate the difference between the experimentally measured ion–ion distances and the ones predicted from Figure 7.7. Assuming we have an accuracy of 0.04 Å in the measurement, would you say that the two sets of ion–ion distances are the same or not? (c) What estimates of the cation–anion distance would you obtain for these four compounds using bonding atomic radii? Are these estimates as accurate as the estimates using ionic radii?
IONIZATION ENERGIES; ELECTRON AFFINITIES (sections 7.4 and 7.5)
7.39 Write equations that show the processes that describe the first, second, and third ionization energies of an aluminum atom. Which process would require the least amount of energy?
7.40 Write equations that show the process for (a) the first two ion-ization energies of lead and (b) the fourth ionization energy of zirconium.
______
7.41 Identify each statement as true or false. If it is false, rewrite it so that it is true: (a) Ionization energies are always negative quantitites. (b) Oxygen has a larger first ionization energy than fluorine. (c) The second ionization energy of an atom is always greater than its first ionization energy.
7.42 (a) Why does Li have a larger first ionization energy than Na? (b) The difference between the third and fourth ionization energies of scandium is much larger than the difference between the third and fourth ionization energies of titanium. Why? (c) Why does Li have a much larger second ionization energy than Be?
______
7.43 (a) What is the general relationship between the size of an atom and its first ionization energy? (b) Which element in the periodic table has the largest ionization energy? Which has the smallest?
7.44 (a) What is the trend in first ionization energies as one proceeds down the group 7A elements? Explain how this trend relates to the variation in atomic radii. (b) What is the trend in first ionization energies as one moves across the fourth period from K to Kr? How does this trend compare with the trend in atomic radii?
______
7.45 Based on their positions in the periodic table, predict which atom of the following pairs will have the smaller first ioniza-tion energy: (a) Cl, Ar; (b) Be, Ca; (c) K, Co; (d) S, Ge; (e) Sn, Te.
7.46 For each of the following pairs, indicate which element has the smaller first ionization energy: (a) Ti, Ba; (b) Ag, Cu; (c) Ge, Cl; (d) Pb, Sb. (In each case use electron configuration and effective nuclear charge to explain your answer.)
______
7.47 Write the electron configurations for the following ions: (a) Fe2+, (b) Hg2+, (c) Mn2+, (d) Pt2+, (e) P3−.
7.48 Write electron configurations for the following ions, and determine which have noble-gas configurations: (a) Cr3+, (b) N3−, (c) Sc3+, (d) Cu2+, (e) Tl+, (f) Au+.
______
7.49 Find three examples of ions in the periodic table that have an electron configuration of nd8 (n = 3, 4, 5…).
7.50 Find three atoms in the periodic table whose ions have an electron configuration of nd6 (n = 3, 4, 5…).
______
7.51 The first ionization energy and electron affinity of Ar are both positive values. (a) What is the significance of the positive value in each case? (b) What are the units of electron affinity?
7.52 If the electron affinity for an element is a negative number, does it mean that the anion of the element is more stable than the neutral atom? Explain.
______
7.53 Although the electron affinity of bromine is a negative quantity, it is positive for Kr. Use the electron configurations of the two elements to explain the difference.
7.54 What is the relationship between the ionization energy of an anion with a 1- charge such as F− and the electron affinity of the neutral atom, F?
______
7.55 Consider the first ionization energy of neon and the electron affinity of fluorine. (a) Write equations, including electron configurations, for each process. (b) These two quantities will have opposite signs. Which will be positive, and which will be negative? (c) Would you expect themagnitudes of these two quantities to be equal? If not, which one would you expect to be larger? Explain your answer.
7.56 Write an equation for the process that corresponds to the electron affinity of the Mg+ ion. Also write the electron configurations of the species involved. What is the magnitude of the energy change in the process? [Hint: The answer is in Table 7.2.]
PROPERTIES OF METALS AND NONMETALS (section 7.6)
7.57 How are metallic character and first ionization energy related?
[7.58] It is possible to define metallic character as we do in this book and base it on the reactivity of the element and the ease with which it loses electrons. Alternatively, one could measure how well electricity is conducted by each of the elements to determine how “metallic” the elements are. On the basis of conductivity, there is not much of a trend in the periodic table: Silver is the most conductive metal, and manganese the least. Look up the first ionization energies of silver and manganese; which of these two elements would you call more metallic based on the way we define it in this book?
______
7.59 Discussing this chapter, a classmate says, “An element that commonly forms a cation is a metal.” Do you agree or disagree? Explain your answer.
7.60 Discussing this chapter, a classmate says, “Since elements that form cations are metals and elements that form anions are nonmetals, elements that do not form ions are metalloids.” Do you agree or disagree? Explain your answer.
______
7.61 Predict whether each of the following oxides is ionic or molecular: SnO2, Al2O3, CO2, Li2O, Fe2O3, H2O. Explain the reasons for your choices.
7.62 Some metal oxides, such as Sc2O3, do not react with pure water, but they do react when the solution becomes either acidic or basic. Do you expect Sc2O3 to react when the solution becomes acidic or when it becomes basic? Write a balanced chemical equation to support your answer.
______
7.63 (a) What is meant by the terms acidic oxide and basic oxide? (b) How can we predict whether an oxide will be acidic or basic based on its composition?
7.64 Arrange the following oxides in order of increasing acidity: CO2, CaO, Al2O3, SO3, SiO2, and P2O5.
______
7.65 Chlorine reacts with oxygen to form Cl2O7. (a) What is the name of this product (see Table 2.6)? (b) Write a balanced equation for the formation of Cl2O7(l) from the elements. (c) Under usual conditions, Cl2O7 is a colorless liquid with a boiling point of 81 °C. Is this boiling point expected or surprising? (d) Would you expect Cl2O7 to be more reactive toward H+(aq) or OH-(aq)? Explain. (e) If the oxygen in Cl2O7 is considered to have the −2 oxidation state, what is the oxidation state of the Cl? What is the electron configuration of Cl in this oxidation state?
[7.66] An element X reacts with oxygen to form XO2 and with chlorine to form XCl4. XO2 is a white solid that melts at high temperatures (above 1000 °C). Under usual conditions, XCl4 is a colorless liquid with a boiling point of 58 °C. (a) XCl4 reacts with water to form XO2 and another product. What is the likely identity of the other product? (b) Do you think that element X is a metal, nonmetal, or metalloid? Explain. (c) By using a sourcebook such as the CRC Handbook of Chemistry and Physics, try to determine the identity of element X.
______
7.67 Write balanced equations for the following reactions: (a) barium oxide with water, (b) iron(II) oxide with perchloric acid, (c) sulfur trioxide with water, (d) carbon dioxide with aqueous sodium hydroxide.
7.68 Write balanced equations for the following reactions: (a) potassium oxide with water, (b) diphosphorus trioxide with water, (c) chromium(III) oxide with dilute hydrochloric acid, (d) selenium dioxide with aqueous potassium hydroxide.
GROUP TRENDS IN METALS AND NONMETALS (sections 7.7 and 7.8)
7.69 Does the reactivity of a metal correlate with its first ionization energy? Explain.
7.70 Silver and rubidium both form +1 ions, but silver is far less reactive. Suggest an explanation, taking into account the ground-state electron configurations of these elements and atomic radii.
______
7.71 (a) Why is calcium generally more reactive than magnesium? (b) Why is calcium generally less reactive than potassium?
7.72 (a) One of the alkali metals reacts with oxygen to form a solid white substance. When this substance is dissolved in water, the solution gives a positive test for hydrogen peroxide, H2O2. When the solution is tested in a burner flame, a lilac-purple flame is produced. What is the likely identity of the metal? (b) Write a balanced chemical equation for reaction of the white substance with water.
7.73 Write a balanced equation for the reaction that occurs in each of the following cases: (a) Potassium metal burns in an atmosphere of chlorine gas. (b) Strontium oxide is added to water. (c) A fresh surface of lithium metal is exposed to oxygen gas. (d) Sodium metal is reacted with molten sulfur.
7.74 Write a balanced equation for the reaction that occurs in each of the following cases: (a) Cesium is added to water. (b) Strontium is added to water. (c) Sodium reacts with oxygen. (d) Calcium reacts with iodine.
______
7.75 (a) As described in Section 7.7, the alkali metals react with hydrogen to form hydrides and react with halogens—for example, fluorine—to form halides. Compare the roles of hydrogen and the halogen in these reactions. How are the forms of hydrogen and halogen in the products alike? (b) Write balanced equations for the reaction of fluorine with calcium and for the reaction of hydrogen with calcium. What are the similarities among the products of these reactions?
7.76 The interior of the planets Jupiter and Saturn are believed to contain metallic hydrogen: hydrogen that is put under such tremendous pressure that it no longer exists as H2 molecules, but instead exists as an extended metallic solid. Predict what properties metallic hydrogen might have compared to “normal” hydrogen in terms of first ionization energy, atomic size, and reactivity.
______
7.77 Compare the elements bromine and chlorine with respect to the following properties: (a) electron configuration, (b) most common ionic charge, (c) first ionization energy, (d) reactivity toward water, (e) electron affinity, (f) atomic radius. Account for the differences between the two elements.
7.78 Little is known about the properties of astatine, At, because of its rarity and high radioactivity. Nevertheless, it is possible for us to make many predictions about its properties. (a) Do you expect the element to be a gas, liquid, or solid at room temperature? Explain. (b) Would you expect At to be a metal, non-metal, or metalloid? Explain. (c) What is the chemical formula of the compound it forms with Na?
______
7.79 Until the early 1960s the group 8A elements were called the inert gases; before that they were called the rare gases. The term rare gases was dropped after it was discovered that argon accounts for roughly 1% of Earth's atmosphere. (a) Why was the term inert gases dropped? (b)What discovery triggered this change in name? (c) What name is applied to the group now?
7.80 (a) Why does xenon react with fluorine, whereas neon does not? (b) Using reference sources such as the CRC Handbook of Chemistry and Physics or online sources, look up the bond lengths of Xe—F bonds in several molecules. How do these numbers compare to the radii of the elements?
______
7.81 Write a balanced equation for the reaction that occurs in each of the following cases: (a) Ozone decomposes to dioxygen. (b) Xenon reacts with fluorine. (Write three different equations.) (c) Sulfur reacts with hydrogen gas. (d) Fluorine reacts with water.
7.82 Write a balanced equation for the reaction that occurs in each of the following cases: (a) Chlorine reacts with water. (b) Barium metal is heated in an atmosphere of hydrogen gas. (c) Lithium reacts with sulfur. (d) Fluorine reacts with magnesium metal.
ADDITIONAL EXERCISES
7.83 Consider the stable elements through lead (Z = 82). In how many instances are the atomic weights of the elements in the reverse order relative to the atomic numbers of the elements? What is the explanation for these cases?
[7.84] We saw in Chapter 6 that the probability of finding an electron in three-dimensional space depends on what orbital it is in. Look back at Figures 6.19 and 6.22, which show the radial probability distribution functions for the s orbitals and contour plots of the 2p orbitals, respectively. (a) Which orbitals, 2s or 2p, have more electron density at the nucleus? (b) How would you modify Slater's rules to adjust for the difference in electronic penetration of the nucleus for the 2s and 2p orbitals?
7.85 (a) If the core electrons were totally effective at screening the valence electrons and the valence electrons provided no screening for each other, what would be the effective nuclear charge acting on the 3s and 3p valence electrons in P? (b) Repeat these calculations using Slater's rules. (c) Detailed calculations indicate that the effective nuclear charge is 5.6+ for the 3s electrons and 4.9+ for the 3p electrons. Why are the values for the 3s and 3p electrons different? (d) If you remove a single electron from a P atom, which orbital will it come from? Explain.
7.86 The size of an atomic nucleus is on the order of 10−15 m. If two protons were able to make a bond, what would you predict the bond length to be?
7.87 As we move across a period of the periodic table, why do the sizes of the transition elements change more gradually than those of the representative elements?
7.88 In the series of group 5A hydrides, of general formula MH3, the measured bond distances are P—H, 1.419 Å; As—H, 1.519 Å; Sb—H, 1.707 Å. (a) Compare these values with those estimated by use of the atomic radii in Figure 7.6. (b) Explain the steady increase in M—H bond distance in this series in terms of the electronic configurations of the M atoms.
7.89 Elements in group 7A in the periodic table are the halogens; elements in group 6A are called the chalcogens. (a) What is the most common oxidation state of the chalcogens compared to the halogens? Can you suggest an explanation for the difference? (b) For each of the following periodic properties, state whether the halogens or the chalcogens have larger values: atomic radii; ionic radii of the most common oxidation state; first ionization energy; second ionization energy.
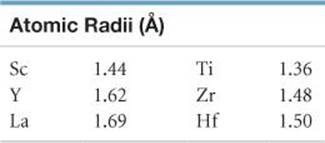
7.90 Note from the following table that the increase in atomic radius in moving from Zr to Hf is smaller than in moving from Y to La. Suggest an explanation for this effect.
[7.91] (a) Which ion is smaller, Co3+ or Co4+? (b) In a lithium ion battery that is discharging to power a device, for every Li+ that inserts into the lithium cobalt oxide electrode, a Co4+ ion must be reduced to a Co3+ ion in order to balance charge. Using the CRC Handbook of Chemistry and Physics or other standard reference, find the ionic radii of Li+, Co3+, and Co4+. Order these ions from smallest to largest. (c) Will the lithium cobalt electrode expand or contract as lithium ions are inserted? (d) Lithium is not nearly as abundant as sodium. If sodium ion batteries were developed that function as lithium ion ones, do you think “sodium cobalt oxide” would still work as the electrode material? Explain. (e) If you don't think cobalt would work as the redox-active partner ion in the sodium version of the electrode, suggest an alternative metal ion and explain your reasoning.
[7.92] The ionic substance strontium oxide, SrO, forms from the reaction of strontium metal with molecular oxygen. The arrangement of the ions in solid SrO is analogous to that in solid NaCl (Figure 2.21): (a) Write a balanced equation for the formation of SrO(s) from its elements. (b)Based on the ionic radii in Figure 7.7, predict the length of the side of the cube in the figure (the distance from the center of an atom at one corner to the center of an atom at a neighboring corner). (c) The density of SrO is 5.10 g/cm3. Given your answer to part (b), how many formula units of SrO are contained in the cube shown here?
7.93 Explain the variation in ionization energies of carbon, as displayed in this graph:

7.94 Group 4A elements have much more negative electron affinities than their neighbors in groups 3A and 5A (see Figure 7.11). Suggest an explanation.
7.95 (a) Use orbital diagrams to illustrate what happens when an oxygen atom gains two electrons. (b) Why does O3− not exist?
[7.96] Use electron configurations to explain the following observations: (a) The first ionization energy of phosphorus is greater than that of sulfur. (b) The electron affinity of nitrogen is lower (less negative) than those of both carbon and oxygen. (c) The second ionization energy of oxygen is greater than the first ion-ization energy of fluorine. (d) The third ionization energy of manganese is greater than those of both chromium and iron.
7.97 The electron affinities, in kJ/mol, for the group 1B and group 2B metals are
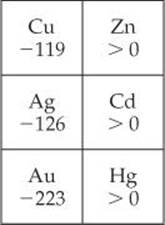
(a) Why are the electron affinities of the group 2B elements greater than zero? (b) Why do the electron affinities of the group 1B elements become more negative as we move down the group? [Hint: Examine the trends in the electron affinity of other groups as we proceed down the periodic table.]
7.98 Hydrogen is an unusual element because it behaves in some ways like the alkali metal elements and in other ways like nonmetals. Its properties can be explained in part by its electron configuration and by the values for its ionization energy and electron affinity. (a) Explain why the electron affinity of hydrogen is much closer to the values for the alkali elements than for the halogens. (b) Is the following statement true? “Hydrogen has the smallest bonding atomic radius of any element that forms chemical compounds.” If not, correct it. If it is, explain in terms of electron configurations. (c) Explain why the ionization energy of hydrogen is closer to the values for the halogens than for the alkali metals. (d) The hydride ion is H−. Write out the process corresponding to the first ionization energy of hydride. (e) How does the process you wrote in part (d) compare to the process for the electron affinity of elemental hydrogen?
[7.99] The first ionization energy of the oxygen molecule is the energy required for the following process:
![]()
7.100 The energy needed for this process is 1175 kJ/mol, very similar to the first ionization energy of Xe. Would you expect O2 to react with F2? If so, suggest a product or products of this reaction.
7.100 The elements of group 4A—carbon, silicon, germanium, tin, and lead—go from nonmetal through metalloid to metal as we go down the column. (a) Predict the order of melting temperature from highest to lowest in this group and justify your logic. (b) Using the CRC Handbok of Chemistry and Physics or other resource, look up the melting points of these elements. How accurate was your prediction?
7.101 Zinc in its 2+ oxidation state is an essential metal ion for life. Zn2+ is found bound to many proteins that are involved in biological processes, but unfortunately Zn2+ is hard to detect by common chemical methods. Therefore, scientists who are interested in studying Zn2+ -containing proteins will frequently substitute Cd2+ for Zn2+, since Cd2+ is easier to detect. (a) On the basis of the properties of the elements and ions discussed in this chapter and their positions in the periodic table, describe the pros and cons of using Cd2+ as a Zn2+ substitute. (b)Proteins that speed up (catalyze) chemical reactions are called enzymes. Many enzymes are required for proper metabolic reactions in the body. One problem with using Cd2+ to replace Zn2+ in enzymes is that Cd2+ substitution can decrease or even eliminate enzymatic activity. Can you suggest a different metal ion that might replace Zn2+ in enzymes instead of Cd2+? Justify your answer.
[7.102] A historian discovers a nineteenth-century notebook in which some observations, dated 1822, were recorded on a substance thought to be a new element. Here are some of the data recorded in the notebook: “Ductile, silver-white, metallic looking. Softer than lead. Unaffected by water. Stable in air. Melting point: 153 °C. Density: 7.3 g/cm3. Electrical conductivity: 20% that of copper. Hardness: About 1% as hard as iron. When 4.20 g of the unknown is heated in an excess of oxygen, 5.08 g of a white solid is formed. The solid could be sublimed by heating to over 800 °C.” (a) Using information in the text and the CRC Handbook of Chemistry and Physics, and making allowances for possible variations in numbers from current values, identify the element reported. (b) Write a balanced chemical equation for the reaction with oxygen. (c) Judging from Figure 7.1, might this nineteenth-century investigator have been the first to discover a new element?
7.103 In April 2010, a research team reported that they had made Element 117. The report has yet to be confirmed. Write out Element 117's ground-state electron configuration, and estimate values for its first ionization energy, electron affinity, atomic size, and common oxidation state based on its position in the periodic table.
7.104 We will see in Chapter 12 that semiconductors are materials that conduct electricity better than nonmetals but not as well as metals. The only two elements in the periodic table that are technologically useful semiconductors are silicon and germanium. Integrated circuits in computer chips today are based on silicon. Compound semiconductors are also used in the electronics industry. Examples are gallium arsenide, GaAs; gallium phosphide, GaP; cadmium sulfide, CdS; cadium selenide, CdSe. (a) What is the relationship between the compound semiconductors' compositions and the positions of their elements on the periodic table relative to Si and Ge? (b) Wo r k e r s in the semiconductor industry refer to “II-VI” and “III-V” materials, using Roman numerals; can you identify which compound semiconductors are II-VI and which are III-V? (c) Suggest other compositions of compound semiconductors based on the positions of their elements in the periodic table.
INTEGRATIVE EXERCISES
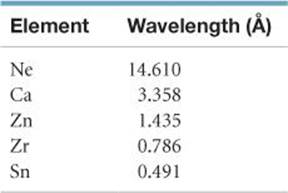
[7.105] Moseley established the concept of atomic number by studying X-rays emitted by the elements. The X-rays emitted by some of the elements have the following wavelengths: (a) Calculate the frequency, n, of the X-rays emitted by each of the elements, in Hz. (b) Using the appropriate graphing program on your computer, plot the square root of n versus the atomic number of the element. What do you observe about the plot? (c) Explain how the plot in part (b) allowed Moseley to predict the existence of undiscovered elements. (d) Use the result from part (b) to predict the X-ray wavelength emitted by iron. (e) A particular element emits X-rays with a wavelength of 0.980 Å. What element do you think it is?
[7.106] (a) Write the electron configuration for Li, and estimate the effective nuclear charge experienced by the valence electron. (b) The energy of an electron in a one-electron atom or ion equals ![]() where Z is the nuclear charge n and n is the principal quantum number of the electron. Estimate the first ionization energy of Li. (c) Compare the result of your calculation with the value reported in Table 7.4 and explain the difference. (d) What value of the effective nuclear charge gives the proper value for the ionization energy? Does this agree with your explanation in (c)?
where Z is the nuclear charge n and n is the principal quantum number of the electron. Estimate the first ionization energy of Li. (c) Compare the result of your calculation with the value reported in Table 7.4 and explain the difference. (d) What value of the effective nuclear charge gives the proper value for the ionization energy? Does this agree with your explanation in (c)?
[7.107] One way to measure ionization energies is ultraviolet photo-electron spectroscopy (UPS, or just PES), a technique based on the photoelectric effect. ![]() (Section 6.2) In PES, monochromatic light is directed onto a sample, causing electrons to be emitted. The kinetic energy of the emitted electrons is measured. The difference between the energy of the photons and the kinetic energy of the electrons corresponds to the energy needed to remove the electrons (that is, the ionization energy). Suppose that a PES experiment is performed in which mercury vapor is irradiated with ultraviolet light of wavelength 58.4 nm. (a) What is the energy of a photon of this light in eV? (b) Write an equation that shows the process corresponding to the first ionization energy of Hg. (c) The kinetic energy of the emitted electrons is measured to be 10.75 eV. What is the first ionization energy of Hg in kJ/mol? (d) Using Figure 7.9, determine which of the halogen elements has a first ionization energy closest to that of mercury.
(Section 6.2) In PES, monochromatic light is directed onto a sample, causing electrons to be emitted. The kinetic energy of the emitted electrons is measured. The difference between the energy of the photons and the kinetic energy of the electrons corresponds to the energy needed to remove the electrons (that is, the ionization energy). Suppose that a PES experiment is performed in which mercury vapor is irradiated with ultraviolet light of wavelength 58.4 nm. (a) What is the energy of a photon of this light in eV? (b) Write an equation that shows the process corresponding to the first ionization energy of Hg. (c) The kinetic energy of the emitted electrons is measured to be 10.75 eV. What is the first ionization energy of Hg in kJ/mol? (d) Using Figure 7.9, determine which of the halogen elements has a first ionization energy closest to that of mercury.
7.108 Mercury in the environment can exist in oxidation states 0, +1, and +2. One major question in environmental chemistry research is how to best measure the oxidation state of mercury in natural systems; this is made more complicated by the fact that mercury can be reduced or oxidized on surfaces differently than it would be if it were free in solution. XPS, X-ray photoelectron spectroscopy, is a technique related to PES (see Exercise 7.107), but instead of using ultraviolet light to eject valence electrons, X-rays are used to eject core electrons. The energies of the core electrons are different for different oxidation states of the element. In one set of experiments, researchers examined mercury contamination of minerals in water. They measured the XPS signals that corresponded to electrons ejected from mercury's 4f orbitals at 105 eV, from an X-ray source that provided 1253.6 eV of energy. The oxygen on the mineral surface gave emitted electron energies at 531 eV, corresponding to the 1s orbital of oxygen. Overall the researchers concluded that oxidation states were +2 for Hg and -2 for O. (a)Calculate the wavelength of the X-rays used in this experiment. (b) Compare the energies of the 4/ electrons in mercury and the 1s electrons in oxygen from these data to the first ionization energies of mercury and oxygen from the data in this chapter. (c) Write out the ground-state electron configurations for Hg2+ and O2+; which electrons are the valence electrons in each case? (d) Use Slater's rules to estimate Zeff for the 4f and valence electrons of Hg2+ and O2−; assume for this purpose that all the inner electrons with (n – 3) or less screen a full +1.
7.109 Consider the gas-phase transfer of an electron from a sodium atom to a chlorine atom:
![]()
(a) Write this reaction as the sum of two reactions, one that relates to an ionization energy and one that relates to an electron affinity. (b) Use the result from part (a), data in this chapter, and Hess's law to calculate the enthalpy of the preceding reaction. Is the reaction exothermic or endothermic? (c) The reaction between sodium metal and chlorine gas is highly exothermic and produces NaCl(s), whose structure was discussed in Section 2.7. Comment on this observation relative to the calculated enthalpy for the aforementioned gas-phase reaction.
[7.110] When magnesium metal is burned in air (Figure 3.6), two products are produced. One is magnesium oxide, MgO. The other is the product of the reaction of Mg with molecular nitrogen, magnesium nitride. When water is added to magnesium nitride, it reacts to form magnesium oxide and ammonia gas. (a) Based on the charge of the nitride ion (Table 2.5), predict the formula of magnesium nitride. (b) Write a balanced equation for the reaction of magnesium nitride with water. What is the driving force for this reaction? (c) In an experiment a piece of magnesium ribbon is burned in air in a crucible. The mass of the mixture of MgO and magnesium nitride after burning is 0.470 g. Water is added to the crucible, further reaction occurs, and the crucible is heated to dryness until the final product is 0.486 g of MgO. What was the mass percentage of magnesium nitride in the mixture obtained after the initial burning? (d) Magnesium nitride can also be formed by reaction of the metal with ammonia at high temperature. Write a balanced equation for this reaction. If a 6.3-g Mg ribbon reacts with 2.57 g NH3(g) and the reaction goes to completion, which component is the limiting reactant? What mass of H2(g) is formed in the reaction? (e) The standard enthalpy of formation of solid magnesium nitride is −461.08 kJ/mol. Calculate the standard enthalpy change for the reaction between magnesium metal and ammonia gas.
7.111 (a) The measured Bi—Br bond length in bismuth tribromide, BiBr3, is 2.63 Å. Based on this value and the data in Figure 7.7, predict the atomic radius of Bi. (b) Bismuth tribromide is soluble in acidic solution. It is formed by treating solid bismuth(III) oxide with aqueous hydrobromic acid. Write a balanced chemical equation for this reaction. (c) While bis-muth(III) oxide is soluble in acidic solutions, it is insoluble in basic solutions such as NaOH(aq). Based on these properties, is bismuth characterized as a metallic, metalloid, or nonmetal-lic element? (d) Treating bismuth with fluorine gas forms BiF5. Use the electron configuration of Bi to explain the formation of a compound with this formulation. (e) While it is possible to form BiF5 in the manner just described, penta-halides of bismuth are not known for the other halogens. Explain why the pentahalide might form with fluorine but not with the other halogens. How does the behavior of bismuth relate to the fact that xenon reacts with fluorine to form compounds but not with the other halogens?
7.112 Potassium superoxide, KO2, is often used in oxygen masks (such as those used by firefighters) because KO2 reacts with CO2 to release molecular oxygen. Experiments indicate that 2 mol of KO2(s) react with each mole of CO2(g). (a) The products of the reaction are K2CO3(s) and O2(g). Write a balanced equation for the reaction between KO2(s) and CO2(g). (b) Indicate the oxidation number for each atom involved in the reaction in part (a). What elements are being oxidized and reduced? (c) What mass of KO2(s) is needed to consume 18.0 g CO2(g)? What mass of O2(g) is produced during this reaction?