CHEMISTRY THE CENTRAL SCIENCE
9 MOLECULAR GEOMETRY AND BONDING THEORIES
9.4 COVALENT BONDING AND ORBITAL OVERLAP
The VSEPR model provides a simple means for predicting molecular geometries but does not explain why bonds exist between atoms. In developing theories of covalent bonding, chemists have approached the problem from another direction, using quantum mechanics. How can we use atomic orbitals to explain bonding and to account for molecular geometries? The marriage of Lewis's notion of electron-pair bonds and the idea of atomic orbitals leads to a model of chemical bonding, called valence-bond theory, in which bonding electron pairs are concentrated in the regions between atoms and nonbonding electron pairs lie in directed regions of space. By extending this approach to include the ways in which atomic orbitals can mix with one another, we obtain an explanatory picture that corresponds to the VSEPR model.
In Lewis theory, covalent bonding occurs when atoms share electrons because the sharing concentrates electron density between the nuclei. In valence-bond theory, we visualize the buildup of electron density between two nuclei as occurring when a valence atomic orbital of one atom shares space, or overlaps, with a valence atomic orbital of another atom. The overlap of orbitals allows two electrons of opposite spin to share the space between the nuclei, forming a covalent bond.
The coming together of two H atoms to form H2 is depicted in ![]() FIGURE 9.13. Each atom has a single electron in a 1s orbital. As the orbitals overlap, electron density is concentrated between the nuclei. Because the electrons in the overlap region are simultaneously attracted to both nuclei, they hold the atoms together, forming a covalent bond.
FIGURE 9.13. Each atom has a single electron in a 1s orbital. As the orbitals overlap, electron density is concentrated between the nuclei. Because the electrons in the overlap region are simultaneously attracted to both nuclei, they hold the atoms together, forming a covalent bond.
The idea of orbital overlap producing a covalent bond applies equally well to other molecules. In HCl, for example, chlorine has the electron configuration [Ne]3s2 3p5. All the valence orbitals of chlorine are full except one 3p orbital, which contains a single electron. This 3p electron pairs with the single 1s electron of H to form a covalent bond (Figure 9.13). Because the other two chlorine 3p orbitals are already filled with a pair of electrons, they do not participate in the bonding to hydrogen. Likewise, we can explain the covalent bond in Cl2 in terms of the overlap of the singly occupied 3p orbital of one Cl atom with the singly occupied 3p orbital of another.
There is always an optimum distance between the two nuclei in any covalent bond. ![]() FIGURE 9.14 shows how the potential energy of a system consisting of two H atoms changes as the atoms come together to form an H2 molecule. When the atoms are infinitely far apart, they do not “feel” each other and so the energy approaches zero. As the distance between the atoms decreases, the overlap between their 1s orbitals increases. Because of the resultant increase in electron density between the nuclei, the potential energy of the system decreases. That is, the strength of the bond increases, as shown by the decrease in the potential energy of the two-atom system. However, Figure 9.14 also shows that as the atoms come closer together than 0.74 Å, the energy increases sharply. This increase, which becomes significant at short internuclear distances, is due mainly to the electrostatic repulsion between the nuclei. The internuclear distance, or bond length, is the distance that corresponds to the minimum of the potential-energy curve. The potential energy at this minimum corresponds to the bond strength. Thus, the observed bond length is the distance at which the attractive forces between unlike charges (electrons and nuclei) are balanced by the repulsive forces between like charges (electron–electron and nucleus–nucleus).
FIGURE 9.14 shows how the potential energy of a system consisting of two H atoms changes as the atoms come together to form an H2 molecule. When the atoms are infinitely far apart, they do not “feel” each other and so the energy approaches zero. As the distance between the atoms decreases, the overlap between their 1s orbitals increases. Because of the resultant increase in electron density between the nuclei, the potential energy of the system decreases. That is, the strength of the bond increases, as shown by the decrease in the potential energy of the two-atom system. However, Figure 9.14 also shows that as the atoms come closer together than 0.74 Å, the energy increases sharply. This increase, which becomes significant at short internuclear distances, is due mainly to the electrostatic repulsion between the nuclei. The internuclear distance, or bond length, is the distance that corresponds to the minimum of the potential-energy curve. The potential energy at this minimum corresponds to the bond strength. Thus, the observed bond length is the distance at which the attractive forces between unlike charges (electrons and nuclei) are balanced by the repulsive forces between like charges (electron–electron and nucleus–nucleus).

![]() FIGURE 9.13 Covalent bonds in H2, HCl, and Cl2 result from overlap of atomic orbitals.
FIGURE 9.13 Covalent bonds in H2, HCl, and Cl2 result from overlap of atomic orbitals.
![]() GO FIGURE
GO FIGURE
On the left part of the curve the potential energy rises above zero. What causes this to happen?
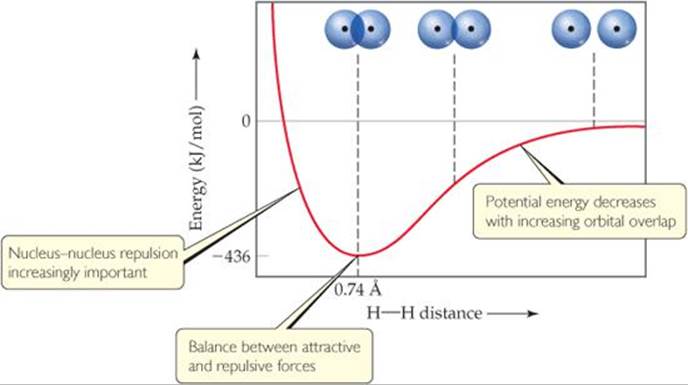
![]() FIGURE 9.14 Formation of the H2 molecule as atomic orbitals overlap.
FIGURE 9.14 Formation of the H2 molecule as atomic orbitals overlap.