CHEMISTRY THE CENTRAL SCIENCE
9 MOLECULAR GEOMETRY AND BONDING THEORIES
9.6 MULTIPLE BONDS
In the covalent bonds we have considered thus far, the electron density is concentrated along the line connecting the nuclei (the internuclear axis). In other words, the line joining the two nuclei passes through the middle of the overlap region. These bonds are called sigma (σ) bonds. The overlap of two s orbitals in H2, the overlap of an s and a p orbital in HCl, the overlap of two p orbitals in Cl2 (all shown in Figure 9.13), and the overlap of a p orbital and an sp hybrid orbital in BeF2 (Figure 9.16) are all σ bonds.
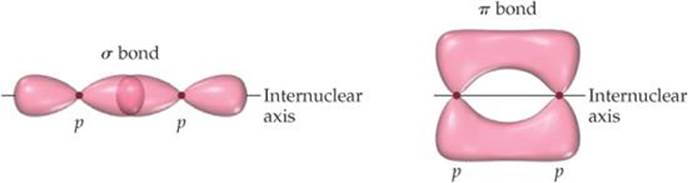
![]() FIGURE 9.21 Comparison of σ and π bonds. Note that the two regions of overlap in the π bond, above and below the internuclear axis, constitute a single π bond.
FIGURE 9.21 Comparison of σ and π bonds. Note that the two regions of overlap in the π bond, above and below the internuclear axis, constitute a single π bond.

![]() FIGURE 9.22 Trigonal-planar molecular geometry of ethylene. The double bond is made up of one C–C σ bond and one C–C π bond.
FIGURE 9.22 Trigonal-planar molecular geometry of ethylene. The double bond is made up of one C–C σ bond and one C–C π bond.
To describe multiple bonding, we must consider a second kind of bond, this one the result of overlap between two p orbitals oriented perpendicularly to the internuclear axis (![]() FIGURE 9.21). This sideways overlap of p orbitals produces a pi (π) bond. A π bond is one in which the overlap regions lie above and below the internuclear axis. Unlike in a σ bond, in a π bond the electron density is not concentrated on the internuclear axis. Although it is not evident in Figure 9.21, the sideways orientation of p orbitals in a π bond makes for weaker overlap. As a result, π bonds are generally weaker than σ bonds.
FIGURE 9.21). This sideways overlap of p orbitals produces a pi (π) bond. A π bond is one in which the overlap regions lie above and below the internuclear axis. Unlike in a σ bond, in a π bond the electron density is not concentrated on the internuclear axis. Although it is not evident in Figure 9.21, the sideways orientation of p orbitals in a π bond makes for weaker overlap. As a result, π bonds are generally weaker than σ bonds.
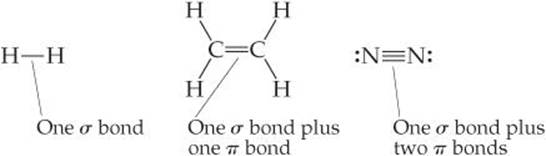
In almost all cases, single bonds are σ bonds. A double bond consists of one σ bond and one π bond, and a triple bond consists of one σ bond and two π bonds:
To see how these ideas are used, consider ethylene (C2H4), which has a C═C double bond. As illustrated by the ball-and-stick model of ![]() FIGURE 9.22, the three bond angles about each carbon are all approximately 120°, suggesting that each carbon atom uses sp2 hybrid orbitals (Figure 9.17) to form σ bonds with the other carbon and with two hydrogens. Because carbon has four valence electrons, after sp2 hybridization one electron in each carbon remains in the unhybridized 2p orbital, which is directed perpendicular to the plane that contains the three sp2 hybrid orbitals.
FIGURE 9.22, the three bond angles about each carbon are all approximately 120°, suggesting that each carbon atom uses sp2 hybrid orbitals (Figure 9.17) to form σ bonds with the other carbon and with two hydrogens. Because carbon has four valence electrons, after sp2 hybridization one electron in each carbon remains in the unhybridized 2p orbital, which is directed perpendicular to the plane that contains the three sp2 hybrid orbitals.
Each sp2 hybrid orbital on a carbon atom contains one electron. ![]() FIGURE 9.23 shows how the C—H σ bonds are formed by overlap of sp2 hybrid orbitals on C with the 1s orbitals on each H atom. We use eight electrons to form these four C—H bonds. The C—Cσ bond is formed by the overlap of two sp2 hybrid orbitals, one on each carbon atom, and requires two more electrons. Thus, ten of the 12 valence electrons in the C2H4 molecule are used to form five σ bonds.
FIGURE 9.23 shows how the C—H σ bonds are formed by overlap of sp2 hybrid orbitals on C with the 1s orbitals on each H atom. We use eight electrons to form these four C—H bonds. The C—Cσ bond is formed by the overlap of two sp2 hybrid orbitals, one on each carbon atom, and requires two more electrons. Thus, ten of the 12 valence electrons in the C2H4 molecule are used to form five σ bonds.
The remaining two valence electrons reside in the unhybridized 2p orbitals, one electron on each carbon. These two orbitals can overlap sideways with each other, as shown in Figure 9.23. The resultant electron density is concentrated above and below the C—C bond axis, which means this is a π bond (Figure 9.21). Thus, the C═C double bond in ethylene consists of one σ bond and one π bond. You should note one point about the carbon p orbitals that form the π bond. It appears from Figure 9.21 that the p orbitals on the two carbons don't overlap sufficiently to form a π bond. The problem is that we can't show the true extent of overlap in the drawing without obscuring other aspects of the figure. Although π bonding of the p orbitals does occur, as pointed out earlier, π bonds are generally weaker than σ bonds.
Although we cannot experimentally observe a π bond directly (all we can observe are the positions of the atoms), the structure of ethylene provides strong support for its presence. First, the C—C bond length in ethylene (1.34 Å) is much shorter than in compounds with C—C single bonds (1.54 Å), consistent with the presence of a stronger C═C double bond. Second, all six atoms in C2H4 lie in the same plane. The 2p orbitals that make up the π bond can achieve a good overlap only when the two CH2 fragments lie in the same plane. If the π bond were absent, there would be no reason for the two CH2 fragments to lie in the same plane. Because π bonds require that portions of a molecule be planar, they can introduce rigidity into molecules.
![]() GO FIGURE
GO FIGURE
Why is it important that the sp2 hybrid orbitals of the two carbon atoms lie in the same plane?
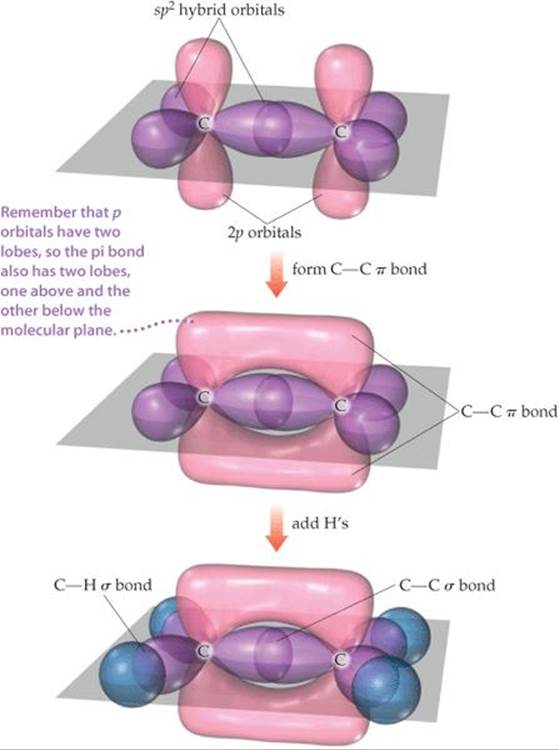
![]() FIGURE 9.23 The orbital structure of ethylene.
FIGURE 9.23 The orbital structure of ethylene.
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
The molecule called diazine has the formula N2H2 and the Lewis structure
![]()
Do you expect diazine to be a linear molecule (all four atoms on the same line)? If not, do you expect the molecule to be planar (all four atoms in the same plane)?
Triple bonds can also be explained using hybrid orbitals. Acetylene (C2H2), for example, is a linear molecule containing a triple bond: H—C≡C—H. The linear geometry suggests that each carbon atom uses sp hybrid orbitals to form σ bonds with the other carbon and one hydrogen. Each carbon atom thus has two unhybridized 2p orbitals at right angles to each other and to the axis of the sp hybrid set (![]() FIGURE 9.24). These p orbitals overlap to form a pair of π bonds. Thus, the triple bond in acetylene consists of one σ bond and two π bonds.
FIGURE 9.24). These p orbitals overlap to form a pair of π bonds. Thus, the triple bond in acetylene consists of one σ bond and two π bonds.
![]() GO FIGURE
GO FIGURE
Based on the models of bonding in ethylene and acetylene, which molecule should have the higher carbon–carbon bond energy?
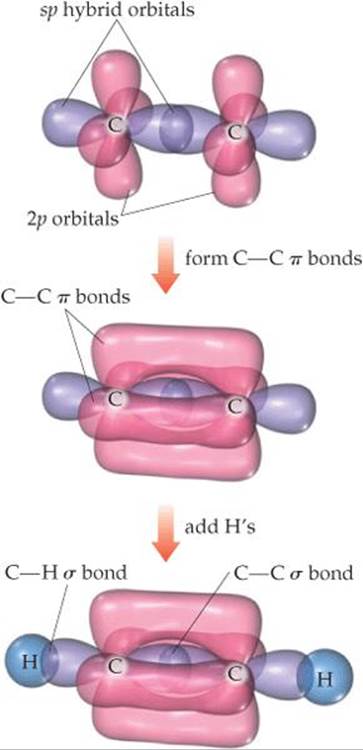
![]() FIGURE 9.24 Formation of two π bonds in acetylene, C2H2.
FIGURE 9.24 Formation of two π bonds in acetylene, C2H2.
Although it is possible to make π bonds from d orbitals, the only π bonds we will consider are those formed by the overlap of p orbitals. These π bonds can form only if unhybridized p orbitals are present on the bonded atoms. Therefore, only atoms having sp2 or sp hybridization can form π bonds. Further, double and triple bonds (and hence π bonds) are more common in molecules made up of period 2 atoms, especially C, N, and O. Larger atoms, such as S, P, and Si, form π bonds less readily.
SAMPLE EXERCISE 9.6 Describing σ and π Bonds in a Molecule
Formaldehyde has the Lewis structure
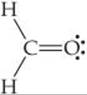
Describe how the bonds in formaldehyde are formed in terms of overlaps of hybrid and unhybridized orbitals.
SOLUTION
Analyze We are asked to describe the bonding in formaldehyde in terms of hybrid orbitals.
Plan Single bonds are σ bonds, and double bonds consist of one π bond and one π bond. The ways in which these bonds form can be deduced from the molecular geometry, which we predict using the VSEPR model.
Solve The C atom has three electron domains around it, which suggests a trigonal-planar geometry with bond angles of about 120°. This geometry implies sp2 hybrid orbitals on C (Table 9.4). These hybrids are used to make the two C—H and one C—O σ bonds to C. There remains an unhybridized 2p orbital on carbon, perpendicular to the plane of the three sp2 hybrids.
The O atom also has three electron domains around it, and so we assume it has sp2 hybridization as well. One of these hybrid orbitals participates in the C—O σ bond, while the other two hold the two nonbonding electron pairs of the O atom. Like the C atom, therefore, the O atom has an unhybridized 2p orbital that is perpendicular to the plane of the molecule. These two orbitals overlap to form a C—O π bond (![]() FIGURE 9.25).
FIGURE 9.25).
PRACTICE EXERCISE
(a) Predict the bond angles around each carbon atom in acetonitrile:
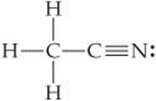
(b) Describe the hybridization at each carbon atom, and (c) determine the number of σ and π bonds in the molecule.
Answers: (a) approximately 109° around the left C and 180° around the right C; (b) sp3, sp; (c) five σ bonds and two π bonds
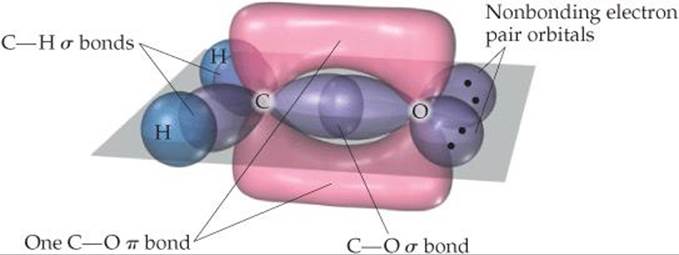
![]() FIGURE 9.25 Formation of σ and π bonds in formaldehyde, H2CO.
FIGURE 9.25 Formation of σ and π bonds in formaldehyde, H2CO.
Resonance Structures, Delocalization, and π Bonding
In the molecules we have discussed thus far in this section, the bonding electrons are localized. By this we mean that the σ and π electrons are associated totally with the two atoms that form the bond. In many molecules, however, we cannot adequately describe the bonding as being entirely localized. This situation arises particularly in molecules that have two or more resonance structures involving π bonds.
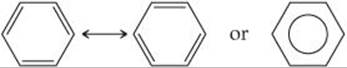
One molecule that cannot be described with localized π bonds is benzene (C6H6), which has two resonance structures: ![]() (Section 8.6)
(Section 8.6)
To describe the bonding in benzene using hybrid orbitals, we first choose a hybridization scheme consistent with the geometry of the molecule. Because each carbon is surrounded by three atoms at 120° angles, the appropriate hybrid set is sp2. Six localized C—C σ bonds and six localized C—H σ bonds are formed from the sp2 hybrid orbitals, as shown in ![]() FIGURE 9.26 (a). This leaves on each carbon a 2p orbital oriented perpendicular to the plane of the molecule. The situation is very much like that in ethylene except we now have six carbon 2p orbitals arranged in a ring [Figure 9.26(b)]. Each unhybridized 2p orbital is occupied by one electron, leaving six electrons to be accounted for by π bonding.
FIGURE 9.26 (a). This leaves on each carbon a 2p orbital oriented perpendicular to the plane of the molecule. The situation is very much like that in ethylene except we now have six carbon 2p orbitals arranged in a ring [Figure 9.26(b)]. Each unhybridized 2p orbital is occupied by one electron, leaving six electrons to be accounted for by π bonding.
![]() GO FIGURE
GO FIGURE
What are the two kinds of σ bonds found in benzene?
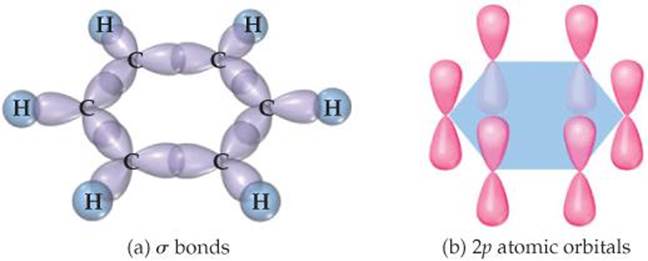
![]() FIGURE 9.26 σ and π bond networks in benzene, C6H6. (a) The σ bond framework. (b) The π bonds are formed from overlap of the unhybridized 2p orbitals on the six carbon atoms.
FIGURE 9.26 σ and π bond networks in benzene, C6H6. (a) The σ bond framework. (b) The π bonds are formed from overlap of the unhybridized 2p orbitals on the six carbon atoms.
We could envision using the unhybridized 2p orbitals to form three localized π bonds. As shown in ![]() FIGURE 9.27, there are two equivalent ways to make these localized bonds, each corresponding to one resonance structure. However, a representation that reflects both resonance structures has the six π electrons “smeared out” among all six carbon atoms, as shown on the right in Figure 9.27. Notice how this combined representation corresponds to the circle-in-a-hexagon drawing we often use to represent benzene. This model leads us to predict that all the carbon-carbon bond lengths will be identical, with a bond length between that of a C—C single bond (1.54 Å) and that of a C═C double bond (1.34 Å). This prediction is consistent with the observed carbon-carbon bond length in benzene (1.40 Å).
FIGURE 9.27, there are two equivalent ways to make these localized bonds, each corresponding to one resonance structure. However, a representation that reflects both resonance structures has the six π electrons “smeared out” among all six carbon atoms, as shown on the right in Figure 9.27. Notice how this combined representation corresponds to the circle-in-a-hexagon drawing we often use to represent benzene. This model leads us to predict that all the carbon-carbon bond lengths will be identical, with a bond length between that of a C—C single bond (1.54 Å) and that of a C═C double bond (1.34 Å). This prediction is consistent with the observed carbon-carbon bond length in benzene (1.40 Å).
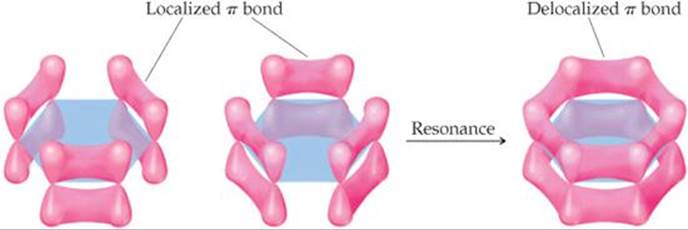
![]() FIGURE 9.27 Delocalized π bonds in benzene.
FIGURE 9.27 Delocalized π bonds in benzene.
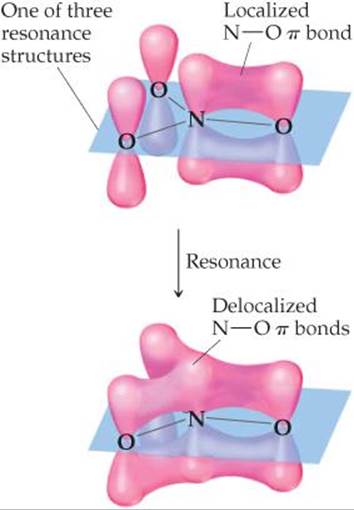
![]() FIGURE 9.28 Localized and delocalized π bonds in NO3–.
FIGURE 9.28 Localized and delocalized π bonds in NO3–.
Because we cannot describe the π bonds in benzene as individual bonds between neighboring atoms, we say that the π bonds are delocalized among the six carbon atoms. Delocalization of the electrons in its π bonds gives benzene a special stability. Delocalization of π bonds is also responsible for the color of many organic molecules. A final important point to remember about delocalized π bonds is the constraint they place on the geometry of a molecule. For optimal overlap of the unhybridized p orbitals, all the atoms involved in a delocalized π bonding network should lie in the same plane. This restriction imparts a certain rigidity to the molecule that is absent in molecules containing only σ bonds (see the “Chemistry and Life” box on vision).
If you take a course in organic chemistry, you will see many examples of how electron delocalization influences the properties of organic molecules.
SAMPLE EXERCISE 9.7 Delocalized Bonding
Describe the bonding in the nitrate ion, NO3–. Does this ion have delocalized π bonds?
SOLUTION
Analyze Given the chemical formula for a polyatomic anion, we are asked to describe the bonding and determine whether the ion has delocalized π bonds.
Plan Our first step is to draw Lewis structures. Multiple resonance structures involving the placement of the double bonds in different locations suggest that the π component of the double bonds is delocalized.
Solve In Section 8.6 we saw that NO3– has three resonance structures:
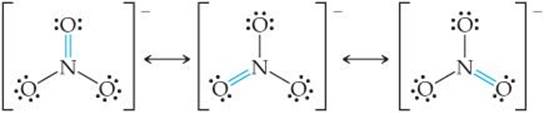
In each structure, the electron-domain geometry at nitrogen is trigonal planar, which implies sp2 hybridization of the N atom. The sp2 hybrid orbitals are used to construct the three N—O σ bonds present in each resonance structure.
The unhybridized 2p orbital on the N atom can be used to make π bonds. For any one of the three resonance structures shown, we might imagine a single localized N—O π bond formed by the overlap of the unhybridized 2p orbital on N and a 2p orbital on one of the O atoms, as shown in ![]() FIGURE 9.28. Because each resonance structure contributes equally to the observed structure of NO3–, however, we represent the π bonding as delocalized over the three N—O bonds, as shown in the figure.
FIGURE 9.28. Because each resonance structure contributes equally to the observed structure of NO3–, however, we represent the π bonding as delocalized over the three N—O bonds, as shown in the figure.
PRACTICE EXERCISE
Which of these species have delocalized bonding: SO3, SO32–, H2CO, O3, NH4+?
Answer: SO3 and O3, as indicated by the presence of two or more resonance structures involving π bonding for each of these molecules
General Conclusions
On the basis of the examples we have seen, we can draw a few helpful conclusions for using hybrid orbitals to describe molecular structures:
1. Every pair of bonded atoms shares one or more pairs of electrons. The lines we draw in Lewis structures represent two electrons each. In every bond at least one pair of electrons is localized in the space between the atoms in a σ bond. The appropriate set of hybrid orbitals used to form the σ bonds between an atom and its neighbors is determined by the observed geometry of the molecule. The correlation between the set of hybrid orbitals and the geometry about an atom is given in Table 9.4.
2. The electrons in σ bonds are localized in the region between two bonded atoms and do not make a significant contribution to the bonding between any other two atoms.
3. When atoms share more than one pair of electrons, one pair is used to form a σ bond; the additional pairs form π bonds. The centers of charge density in a π bond lie above and below the internuclear axis.
4. Molecules with two or more resonance structures can have π bonds that extend over more than two bonded atoms. Electrons in π bonds that extend over more than two atoms are said to be “delocalized.”
 CHEMISTRY AND LIFE
CHEMISTRY AND LIFE
THE CHEMISTRY OF VISION
Vision begins when light is focused by the lens of the eye onto the retina, the layer of cells lining the interior of the eyeball. The retina contains photoreceptor cells called rods and cones (![]() FIGURE 9.29). The rods are sensitive to dim light and are used in night vision. The cones are sensitive to colors. The tops of the rods and cones contain a molecule called rhodopsin, which consists of a protein, opsin, bonded to a reddish purple pigment called retinal. Structural changes around a double bond in the retinal portion of the molecule trigger a series of chemical reactions that result in vision.
FIGURE 9.29). The rods are sensitive to dim light and are used in night vision. The cones are sensitive to colors. The tops of the rods and cones contain a molecule called rhodopsin, which consists of a protein, opsin, bonded to a reddish purple pigment called retinal. Structural changes around a double bond in the retinal portion of the molecule trigger a series of chemical reactions that result in vision.
We know that a double bond between two atoms is stronger than a single bond between the same atom, but our recent discussions allow us to appreciate another aspect of double bonds: the rigidity they introduce into molecules.
Imagine rotating one—CH2 group in ethylene relative to the other—CH2 group, as in ![]() FIGURE 9.30. This rotation destroys the overlap of p orbitals, breaking the π bond, a process that requires considerable energy. Thus, the presence of a double bond restricts bond rotation in a molecule. In contrast, molecules can rotate almost freely around the bond axis in single (σ) bonds because this motion has no effect on the orbital overlap for a σ bond. This rotation allows molecules with single bonds to twist and fold almost as if their atoms were attached by hinges.
FIGURE 9.30. This rotation destroys the overlap of p orbitals, breaking the π bond, a process that requires considerable energy. Thus, the presence of a double bond restricts bond rotation in a molecule. In contrast, molecules can rotate almost freely around the bond axis in single (σ) bonds because this motion has no effect on the orbital overlap for a σ bond. This rotation allows molecules with single bonds to twist and fold almost as if their atoms were attached by hinges.
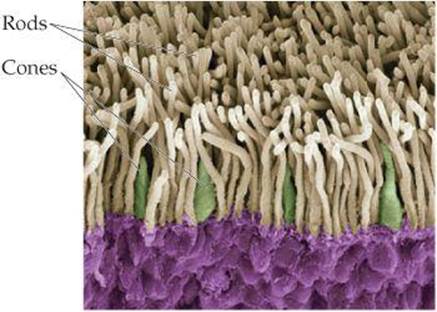
![]() FIGURE 9.29 Inside the eye. A color-enhanced scanning electron micrograph of the rods and cones in the retina of the human eye.
FIGURE 9.29 Inside the eye. A color-enhanced scanning electron micrograph of the rods and cones in the retina of the human eye.
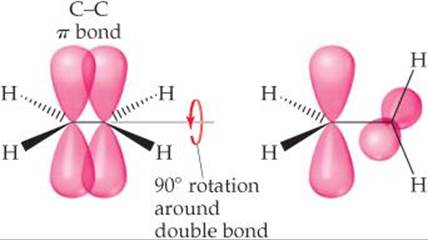
![]() FIGURE 9.30 Rotation about the carbon-carbon double bond in ethylene breaks the π bond.
FIGURE 9.30 Rotation about the carbon-carbon double bond in ethylene breaks the π bond.
Our vision depends on the rigidity of double bonds in retinal. In its normal form, retinal is held rigid by its double bonds. Light entering the eye is absorbed by rhodopsin, and the energy is used to break the π-bond portion of the double bond shown in red in ![]() FIGURE 9.31. The molecule then rotates around this bond, changing its geometry. The retinal then separates from the opsin, triggering the reactions that produce a nerve impulse that the brain interprets as the sensation of vision. It takes as few as five closely spaced molecules reacting in this fashion to produce the sensation of vision. Thus, only five photons of light are necessary to stimulate the eye.
FIGURE 9.31. The molecule then rotates around this bond, changing its geometry. The retinal then separates from the opsin, triggering the reactions that produce a nerve impulse that the brain interprets as the sensation of vision. It takes as few as five closely spaced molecules reacting in this fashion to produce the sensation of vision. Thus, only five photons of light are necessary to stimulate the eye.
The retinal slowly reverts to its original form and reattaches to the opsin. The slowness of this process helps explain why intense bright light causes temporary blindness. The light causes all the retinal to separate from opsin, leaving no molecules to absorb light.
RELATED EXERCISES: 9.108 and 9.112
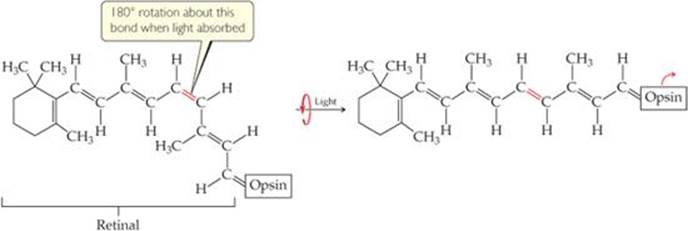
![]() FIGURE 9.31 The rhodopsin molecule, the chemical basis of vision. When rhodopsin absorbs visible light, the π component of the double bond shown in red breaks, allowing rotation that produces a change in molecular geometry before the π bond re-forms.
FIGURE 9.31 The rhodopsin molecule, the chemical basis of vision. When rhodopsin absorbs visible light, the π component of the double bond shown in red breaks, allowing rotation that produces a change in molecular geometry before the π bond re-forms.
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
When two atoms are bonded by a triple bond, what is the hybridization of the orbitals that make up the σ-bond component of the bond?