CHEMICAL BIOLOGY
Neurotransmission, Measuring Chemical Events in
Christopher B. Jacobs and Trisha L. Vickrey Department of Chemistry, University of Virginia, Charlottesville, Virginia
B. Jill Venton Department of Chemistry, Neuroscience Graduate Program, University of Virginia, Charlottesville, Virginia
doi: 10.1002/9780470048672.wecb383
Measuring chemical events in neurotransmission is challenging because fast measurements of small amounts of neurotransmitters and neuromodulators are needed to track the dynamics of chemical changes accurately. In this article, we outline the basics of three popular methods for measuring neurochemical changes: electrophysiology, microdialysis, and electrochemistry. Electrophysiological techniques measure changes in membrane potentials associated with neurotransmission. These methods are often used to measure receptor-gated ion channel currents and are popular for neuropharmacology studies. Microdialysis is a sampling technique that can be used to monitor basal levels of neurotransmitters directly. When coupled to a separation technique, microdialysis is advantageous because it can be used to detect virtually any compound in the brain. Electrochemical techniques are popular because microelectrodes allow rapid, direct detection of neurotransmitters with minimal tissue disturbance. Although the analytes must be electroactive, electrochemistry has been used successfully to monitor neurochemical changes in various preparations, from single cells to behaving animals. Future research in monitoring neurochemical events will include improving the temporal resolution, spatial resolution, and selectivity of measurements.
Neurotransmission is the transfer of an informational signal, a chemical messenger, between two neurons. The traditional picture of synaptic transmission is shown in Fig. 1. A terminal from one neuron forms a synapse with a dendrite of another neuron. After the neurotransmitter is synthesized in the perikaryon of the terminal, it is packaged into specialized synaptic vesicles. The vesicles range in size from about 50 nm to 100 nm and can store between 3000 and 30,000 neurotransmitter molecules (1). An action potential propagating to the terminal changes the cell membrane potential and can induce the fusion of vesicles to the synaptic membrane. This fusion results in exocytosis, the coordinated release of transmitter molecules into the synapse (Fig. 1). Transmitters can then bind to postsynaptic receptors and activate signaling pathways through GTP-binding proteins or open gated ion channels, which leads to localized changes in membrane potential (2). Membrane potentials, ionic currents, and action potentials can be measured using electrophysiology techniques.
Released neurotransmitters can have fates other than binding postsynaptic receptors. Transmitters can bind to presynaptic receptors that act as a feedback loop to regulate additional release of the chemical messengers. Transporters take up neurotransmitters back into the neuron and clear them from the extracellular space. Neurotransmission can also be ended by enzymatic degradation of transmitters, although this process is kinetically slower than uptake. Although the traditional picture of neurotransmission is of short-range signaling, neurotransmitters can also diffuse out of the synapse and act at distal targets, which allows longer distance signaling (3). This process is called volume transmission, and extracellular detection methods such as microdialysis and microelectrodes detect these extrasynaptic concentrations.

Figure 1. General concept representation of synaptic transmission. Vesicles that contain neurotransmitter molecules dock to the cell membranes and release their contents into the synapse by exocytosis. Neurotransmitters can diffuse across the synapse and bind to postsynaptic receptors or can diffuse out of the synapse. This extrasynaptic neurotransmitter can bind to presynaptic receptors, diffuse away and activate receptors on distal neurons, or be cleared from the extracellular space by transporters.
Electrophysiology
Electrophysiological techniques measure changes in potentials associated with neurotransmission. The potential of the neuronal membrane is controlled by ionic concentrations, which are regulated by the active transport of ions. As postsynaptic dendrites receive neurotransmitter signals, receptor-gated Na+ and Ca+ channels are activated, which allows influx down the ionic gradient. Membrane depolarization occurs as cations enter the cell. Once the depolarization reaches a certain threshold, successive Na+ and Ca2+ ion channels open along the axon in a domino fashion to propagate the electrical signal. This propagation of voltage is called an action potential, and it will not occur unless a threshold depolarization is reached. After maximal depolarization, Na+ and Ca2+ ion channels begin to close, and simultaneously, K+ ion channels open and K+ effluxes out of the nerve cell. This process reverses the polarization, and because of the length of time the K+ channels stay open, the abundance of cations outside the cell causes a hyperpolarization of the membrane potential.
Transmembrane movement of ions is controlled by gated ion channels and can be represented by a simple RC circuit, in which the ion channels act as resistors and the cell membrane acts as a capacitor. Hodgkin and Huxley, who won the 1963 Nobel Prize for Physiology or Medicine for their work, quantified these transmembrane voltage changes in the axon of a giant squid and determined the time-dependent behavior of Na+ and K+ channels. Current electrophysiological studies measure neurogenic changes through various intracellular and extracellular techniques. Although these techniques are diverse, common intracellular techniques include the two-electrode voltage clamp, patch clamp, and whole cell methods that measure ion channel currents and common extracellular techniques that measure voltage changes outside cells.
Two-electrode voltage clamp
Voltage clamp methods typically involve two micropipette electrodes that impale the cell; one electrode measures voltage changes, whereas the other applies a stimulating voltage. Transmembrane voltage is clamped at a constant voltage eliminating capacitive current, and allowing conductance to be measured, which is proportional to voltage. The clamped voltage then is varied in a stepwise fashion with each step resulting in a change in ion current, which is measured. For this reason, voltage clamp techniques are useful in studying voltage-gated ion channels. In a typical experiment, voltage steps are applied to an oocyte or insect muscle cell, and the effects of the addition of pharmacological agents on ion channel currents are tested. For example, Fig. 2a shows voltage clamp recordings of inward calcium channel currents in parasitic nematode muscle cells after a series of depolarizing steps (4). The current versus voltage graph shows the maximal current was induced by a step to 0V. The study showed that bathing a voltage-clamped cell in the nematode neuropeptide, AF2, increased the inward calcium currents, which suggests that the neuromuscular function of the parasitic nematode was modulated by AF2. Such studies can be useful for identifying possible target sites for drugs that act at voltage-gated channels.

Figure 2. a) Two-electrode voltage clamp experiment. The cell potential was held at -35 mV, depolarizing steps from -35 to +20 mV were made (top left), and resultant inward currents from Ca2+ channels were measured for each step (bottom left). The right graph shows an I-V curve for nematode muscle Ca2+ ion channels. (Data reprinted from Ref. (4) by permission of Macmillan Publishers Ltd.) b) Whole cell experiment. Representative trace of an individual EPSC before (left) and after (right) application of an adenosine A2A receptor agonist CGS 21680. The agonist causes the ESPC amplitude to decrease. (Data reprinted from Ref. (5) by permission of Elsevier.) c) Inside-out experiment. A continuous single-channel current is shown from in an inside-out membrane patch of a rat cultured hippocampal neuron before and after addition of diazepam. The top trace shows currents induced by 5nM GABA. The bottom four traces show currents induced after a 1 nM solution of diazepam is introduced. A gradual increase in single-channel current amplitude occurs. (Data reprinted from Ref. (8) by permission of Macmillan Publishers Ltd.)
Patch clamp
Some of the most popular electrophysiology methods currently used are patch clamp techniques, in which individual ion channels can be studied. Unlike the two-electrode methods described above, these methods use a single electrode to measure voltage changes. A glass pipette electrode with a flat, open tip is placed onto a cell, and piece of membrane that contains an ion channel is “sucked” into the pipette tip. This action allows the direct current of ions moving through channels to be measured. Although the pipette tip has a high resistance, negative pressure is applied to form a seal with a gigaohm resistance, which is commonly referred to as a gigaseal. The pipette tip is usually filled with a solution that approximates intracellular fluid. Two types of experiments can be performed once the pipette electrode is in place: voltage clamp or current clamp. Similar to two-electrode voltage clamp methods described above, in voltage clamp mode the membrane potential is held at a constant voltage, and current through the ion channels is measured. In current clamp mode, the current is kept constant, and voltage changes are measured. To identify which types of channels contribute to the signal, electrophysiology techniques are commonly used in conjunction with pharmacology experiments, in which drugs are applied to block specific channels. Several configurations of this method correspond to whole cell, outside-out and inside-out models (Fig. 3).

Figure 3. Schematic of a) whole cell, b) outside-out, and c) inside-out patch clamp methods.
Whole cell model
In the whole cell model, a gigaseal is formed as the pipette is attached to the cell, and then a more dynamic suction is applied, which causes the interior of the cell to be sucked into the pipette tip (Fig. 3a). This action allows current and conductance of the entire cell to be measured. Therefore, the whole cell model measures changes caused by many ion channels on the entire cell membrane. Additionally, the liquid content of the cell will mix and equilibrate with the solution in the pipette, which allows pharmacological agents to be administered into the cell. Of the patch clamp techniques, the whole cell method is the most common and can be used to determine how pharmacological agents affect the total conductance of neurons.
An example of a whole cell study is shown in Fig. 2b. Excitatory postsynaptic currents (EPSCs), which are caused by the movement of positive ions into the postsynaptic cell through glutamate-mediated channels, were monitored. This study examined whether adenosine receptor activation could modulate EPSCs. Whole cell patch clamp was performed on neurons in striatal rat brain slices. Figure 2b shows an EPSC evoked by electrical stimulation before and after application of an A2A adenosine receptor agonist. The decreased amplitude of the EPSC after the adenosine receptor agonist is applied suggests that adenosine receptor activation inhibits glutamate channel-mediated EPSCs (5). Many researchers also study inhibitory postsynaptic currents (IPSCs) mediated by gamma-aminobutyric acid-(GABA-)-gated chloride channels. For example, whole cell patch clamp was used to differentiate two different types of IPSCs in substantia gelatinosa neurons of the mouse spinal cord (6). IPSCs with a fast decay had a different pharmacological profile than those with a slow decay. Whole cell methods continue to be popular for neuropharmacology studies because they allow researchers to understand the complex actions and regulation of receptors gating ion channels.
Outside-out model
In the outside-out model, the pipette is attached to the entire cell as in the whole cell model, followed by a sharp pull that causes the cell membrane to break and reseal with the pipette tip (Fig. 3b). With the extracellular region exposed, channel activity as a response to different external stimuli can be probed. This configuration is less common than the inside-out method. Using an outside-out method, single-channel opening activity has been recorded while various neurotransmitters were released. For example, this patch clamp method was used as a detector for capillary electrophoresis separations of GABA, glutamate, and NMDA (7).
Inside-out model
In the inside-out model, gigaseal formation is followed by a sharp pull, which detaches the cell membrane and exposes the inner membrane of the cell to the bathing solution. The portion of the membrane that was on the inside of the cell is then on the outside of the pipette, whereas the outer portion is in equilibrium with the fluid inside the pipette (Fig. 3c). This configuration is useful for studying the effects of intracellular molecules or drugs on individual ion channels and has been used in pharmacology experiments to study ligand-gated ion channel activity. For example, benzodiazepines, which are used to treat anxiety, are thought to augment synaptic inhibition in the central nervous system. Inside-out patch clamp was used to study the effect of the benzodiazepine, diazepam, on single-channel conductance (8). Diazepam caused a seven-fold increase in the conductance of GABAA chloride channels from rat cultured hippocampal neurons (Fig. 2c). This study demonstrates that the inside-out method can provide information about the effect of drugs on the current of a particular ion channel. Inside-out patch clamp has also been used to study pain sensory transduction in rat ganglion neurons through capsaicin-activated ion channels (9). Capsaicin is known to activate certain cation channels and cause severe pain. One study used patch clamp to determine that 12-(S)-hydroxyeicosatetraenoic acid (HPETE), a lipoxygenase product, was an endogenous activator of capsaicin channels. The inside-out technique is widely used to study various channels, both inhibitory and excitatory, and various preparations from retinal neurons to central neurons. For example, inside-out patch clamp has been used to study the photoresponse of rat intrinsically photosensitive retinal ganglion cells (ipRGCs), which are photoreceptors that control pupil response (10). In this study, inside-out recordings showed that ipRGCs are photosensitive. This study gives insight into the complex cascade of events that lead to vision.
Extracellular methods
With intracellular methods, the change in voltage caused by the fluctuation of ions across the cell membrane is measured. However, transmembrane ion concentration fluxes also generate an electric field in the extracellular space, and these changes in electrical activity can be measured by extracellular recordings. Both invasive and noninvasive techniques are routinely used. Extracellular techniques can be used in many preparations, including awake, behaving animals.
In a typical invasive setup, a wire electrode or an array of wires is implanted into the brain, and changes in potential are measured. Electrodes that are directly next to a neuron will record voltage changes when that neuron fires. This setup has been used to measure neuronal firing patterns in animals performing complex behaviors, such as drug self-administration (11). These studies have shown that dopamine neurons fire as predictive signals of reward and will fire in response to cues that have been previously associated with rewards (12). Multielectrode arrays have also been used to map the response of different neurons to external stimulations. For example, Nicolelis et al. have used multichannel electrodes to map tactile responses of an anesthetized rat (13). Figure 4 demonstrates the neuronal response to stimulation of the infraorbital nerve, which carries information from the whisker’s mechanoreceptors to the brain. Typical data is shown, in which firing patterns before and after the stimulus (dashed line, time 0) are given for multiple trials (top of figures) and then binned to get average firing rates (histograms). The results show that the hippocampal neurons have a longer latency to fire but are activated for longer than the neurons in the somatosensory cortex and thalamic ventral posteromedial nucleus. Such studies help identify and interpret the neuronal pathways that process tactile stimulation.
In a noninvasive setup, surface electrodes are placed on the surface of the skin and can be used to study individual or multiple neurons. Additionally, many studies look at motor units, which are α motor neurons and all muscle fibers that the neuron innervates. For example, stable recordings of single motor unit potentials can be made using electromyography (14). Surface electrodes are of interest in electroencephalography (EEG) measurements, in which electrodes are placed on the scalp. Noninvasive EEG measurements are particularly useful in human studies. For example, EEG recordings have been used to study adenosinergic neurotransmission in homeostatic sleep-wake regulation in humans (15). These studies found that adenosinergic neurotransmission plays a role in non-rapid-eye-movement sleep homeostasis.
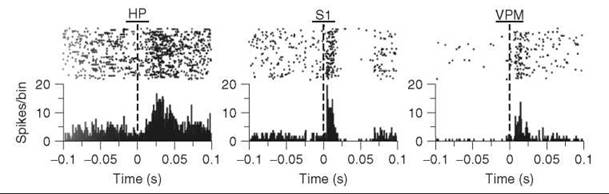
Figure 4. Electrically evoked single-unit tactile responses recorded with microwire multielectrode arrays in rat hippocampus CA1, primary somatosensory cortex, and ventral posteromedial nucleus brain regions. (Upper) Raster plot of single-unit spikes before and after electrical current stimulation to whiskers. Each row is a separate trial. (Lower) Summed activity for all trials in 1-ms bins that demonstrate a response to electrical stimulation. The graphs show different latencies in firing for the three different regions. [Data reprinted from Ref. (13). Copyright (2007) National Academy of Sciences, U.S.]
Summary and future directions
Intracellular patch clamp methods provide a sensitive, high- resolution approach to studying ion channels. Patch clamp studies are particularly useful in pharmacological studies, such as identifying receptor agonists and antagonists. Studies can be performed in brain slices or in vivo. However, several pervasive problems with patch clamping exist. Patch clamping is inherently an invasive technique, and morphological changes in the cell can occur during the clamping process that interfere with the overall function of the ion channel investigated (16). Although patch clamp methods are robust and have provided information about the function of ion channels, they are labor-intensive, require a high degree of technical skill, and are low-throughput. Therefore, a research topic of current interest is developing automated, high-throughput patch clamp methods. For example, a planar patch clamp electrode, which uses a planar substrate rather than a pipette to determine current for whole cells, has been developed. Planar patch clamp requires less skill and allows higher throughput measurements to be made (17). Interest in developing methods to screen ion channels as drug targets has led to the commercial availability of automated planar patch clamp systems (18). Additional development of these high-throughput methods is expected to lead to widespread adoption of these techniques.
With extracellular measurements, action potential firing adjacent to an electrode gives information about neuron output and synaptic input. These methods are good for measuring neuronal activity of awake, behaving animals. The use of implantable electrodes provides high resolution of single neurons, whereas nonimplantable electrodes sample a larger surface area, which causes them to have a lower resolution. However, noninvasive measurements are the most useful for higher order animals and humans. Signals from extracellular recordings are much smaller and harder to detect than with intracellular methods. For example, the number or type of neurons that are firing at a given time can be difficult to determine (19). Additionally, action potentials are often obscured or unsynchronized, and extracellular recordings can be a summation of multiple neuronal signals. However, with the development of better computer programs and bioinformatics capabilities for data analysis, these problems can be overcome (20). Additional integration of both intracellular and extracellular methods will provide a more holistic picture of neurotransmission.
Microdialysis
Microdialysis has served as a powerful tool in the direct measurement of cerebral neurotransmitters. It can be used as a qualitative technique to monitor changes in neurotransmitters or as a quantitative technique to determine actual neurotransmitter concentrations such as basal levels. Measurements are made using a probe sheathed with a semipermeable membrane, which allows extracellular molecules of a low molecular weight, such as neurotransmitters, to pass but excludes larger molecules such as proteins. The probe is perfused with artificial cerebral spinal fluid, and molecules diffuse across the membrane according to their concentration gradients. Fluid fractions are collected at the probe outlet. Fractions are typically analyzed using separation techniques, such as high-performance liquid chromatography (HPLC) or capillary electrophoresis (CE). Microdialysis can provide both highly selective and sensitive analysis of neurochemical changes, but the temporal resolution has been limited traditionally to 5-20 minutes (21, 22). Continuous sampling of chemical events allows processes such as drug metabolism and neurotransmitter release to be studied. Microdialysis also is useful for studying neurotransmission in various preparations, including in behaving animals.
Microdialysis Theory
In microdialysis, a solution isotonic to that being sampled is perfused through an inlet tube of the probe (Fig. 5). A concentration gradient is established on each side of the membrane between the perfusate and the sampling region. This gradient causes neurochemicals from the sampling region to diffuse through the membrane, and the compounds are collected for analysis at the outlet of the probe. Analyte removal from the sampling region keeps the concentration gradient intact and allows continual sampling of the extracellular space. This process is highly dependent on flow rate of perfusion.
At typical flow rates, the concentration in the dialysate, Cout, is less than the actual concentration in the extracellular fluid, Cext (23). The ratio of Cout/Cext is defined as relative recovery, R, and must be considered for probe calibration and sampling optimization. In vitro, R is easily calculated because the dialysate and the extracellular fluid are homogenous; therefore, probe calibration is easily obtained. However, in in vivo studies, calculation of R is difficult because of the active removal of neurotransmitters by uptake and tortuosity. Movement of analytes is impeded by tissue that surrounds the probe, and this movement cannot be easily accounted for with in vitro calibrations. Therefore, the most common method to determine concentrations in vivo is the zero-net flux method, in which known analyte concentrations are added to the perfusate (Cin), and then the analyte concentration is measured at the probe outlet (Cout). The difference between analyte concentration at the inlet and outlet is used to establish the actual analyte concentration in the tissue, and the relative recovery rate can be calculated. This calibration method can be used to estimate basal levels of neurotransmitters. For example, the zero-net flux method has been used to determine that basal concentrations of dopamine are approximately 1-3.5 nM (24, 25). Although basal level concentrations of neurotransmitters can be determined with this method, the contribution of tissue damage to the microdialysis signal cannot be neglected. Because microdialysis is an invasive technique, a traumatized layer of tissue will be next to the probe, which influences the rate of neurotransmitter release and uptake. Bungay et al. have suggested in the case of dopamine that trauma leads to an underestimation of extracellular dopamine concentration and have proposed a quantitative model to correct for that (23).

Figure 5. Microdialysis probe. Artificial cerebral spinal fluid is perfused through the inlet, and small molecules can diffuse across the membrane. Fluid is collected at the outlet and analyzed.
Microdialysis coupled to high-performance liquid chromatography
Detection of neurotransmitters in microdialysate samples has been achieved traditionally by coupling microdialysis sampling to highly sensitive analysis methods such as high-performance liquid chromatography (HPLC). HPLC is a type of column chromatography, which separates the collected mixture of analytes and resolves them into individual components. Therefore, multiple chemical species can be identified in a given sample. Monitoring changes in concentrations over time gives insight into a wide range of processes such as transporter function, effects of pharmacological agents, and behaviorally evoked neurotransmitter changes.
Glutamate is the most abundant excitatory neurotransmitter in the nervous system. Under normal conditions, glutamate transporters remove extracellular glutamate into glial cells. However, during disease or ischemia, a lack of oxygen delivery to cells, excess glutamate can accumulate and result in excitotoxicity. Microdialysis studies have played an important role in understanding the relationship between glutamate levels and ischemic events. Glutamate has been shown to increase during a stroke, which contributes directly to neuron damage and additional vulnerability to ischemia (26, 27). Subsequently, methods to reduce glutamate release during an ischemic event have been of interest. Recent microdialysis studies indicate that spinal cord necrosis can be reduced by administering magnesium sulfate to the area experiencing ischemia (28). Figure 6 shows typical data where microdialysis samples were collected and glutamate concentration quantitated every 10 minutes. Intracerebral occlusion of the aortic artery caused ischemia and glutamate levels to increase (open circles). However, when MgSO4 was administered, glutamate did not rise during ischemia, similar to the sham control group. Therefore, microdialysis gives information about how pharmacological treatments could be used to prevent glutamate excitotoxic damage during ischemia.
The neurotransmitter dopamine is of particular interest because of the role it plays in brain processes related to movement, cognition, reward, and addiction. Changes in basal levels of dopamine have been monitored using microdialysis to understand the effects of drugs of abuse, such as cocaine, on neurotransmission (29). Dopamine levels have also been measured in different behavioral paradigms. For example, using the zero-net flux method, Chefer et al. have demonstrated an increase in basal dopamine levels in the nucleus accumbens following cocaine stimulation (30). Moreover, they coupled locomotor response to novelty with dopamine levels in response to cocaine. Male rats were screened as either high responders or low responders to environmental stimuli, and high responders showed the greatest increase in dopamine after cocaine. More studies have investigated the effect of drugs, such as cocaine, morphine, and amphetamine, on dopamine levels to determine genetic factors in behavior response and drug abuse (29).

Figure 6. Effects of intrathecal magnesium sulfate on ischemia-induced release of glutamate in the dialysate collected from the spinal cord of rabbits. Data are expressed as percentage of the mean baseline levels in each group. Aortic artery occlusion to cause ischemia results in an increase in glutamate in the placebo group (open circles), but administration of MgSO4 before ischemia is induced prevents the increase (triangles). A sham group in which ischemia is not induced is also shown (circles). (Data reprinted from Ref. (28) by permission of Lippincott, Williams, and Wilkins.)
Microdialysis coupled to capillary electrophoresis
Recently, several groups have used capillary electrophoresis (CE) instead of HPLC as an analysis method for microdialysis samples. The chief advantage of CE is that high-speed separations of very small sample volumes can be achieved. The improved temporal resolution allows samples to be analyzed every 10 seconds, which facilitates better understanding of the dynamics of neurotransmitter changes during behavior. This method has enabled the online analysis of microdialysis samples using capillary electrophoresis and eliminated the need to store fractions. The disadvantage is that samples must be derivatized to make them fluorescent so that highly sensitive laser-induced fluorescence can be used for detection.
Microdialysis coupled to CE was used to detect changes in amino acids, such as glutamate, during exposure of rats to a predator fox odor (31). Figure 7 shows that large changes in glutamate were observed after presenting the fox odor, particularly in a subset of rats that were high responders. These changes lasted only a few minutes. The data are compared with a similar experiment that measured glutamate changes in response to the presentation of the fox odor with typical microdialysis-HPLC with only 10-minute temporal resolution (32). The magnitude and duration of the glutamate change are more accurately tracked with the higher temporal resolution CE method. Microdialysis-CE has been used to study amino acid neurotransmitters during learning (33) and catecholamines such as dopamine and norepinephrine during the sleep-wake cycle (34). Future research in this field includes miniaturizing the CE detection on a chip (35) to make the technique more amenable to wide-scale implementation.

Figure 7. Glutamate changes in the nucleus accumbens after presentation of the predator fox odor 2,5-dihydro-2,4,5-trimethylthiazoline. Data are shown as a percentage of basal levels, and presentation of the fox odor causes glutamate to increase. Microdialysis coupled with CE data (solid circles) are compared with previous studies of microdialysis coupled with HPLC (open triangles). Microdialysis coupled to CE provided information not previously found with HPLC because of increased time resolution. (Data adapted from Ref. (31) by permission from Blackwell Publishing.)
Summary and future directions
Although microdialysis is sensitive and selective, it is an invasive technique that causes trauma and tissue damage, such as edema and adulteration of the blood-brain barrier and glucose metabolism in areas near probe insertion. Consequently, interpretation of results garnered from microdialysis has been controversial. Several studies indicate cerebral blood flow and glucose metabolism recovery within 24 hours (27), and many studies delay measurements after probe implantation to allow tissue recovery. However, recent studies that compare dopamine levels at the insertion point and 1mm adjacent to this area indicate permanent changes in tissue because of trauma (36). Furthermore, neuronal loss and tissue disruption have been observed through light and electron microscopic analysis (37).
Nevertheless, microdialysis measurements agree well with other methods used to measure neurochemical changes such as microelectrode techniques (38). For example, pulse voltammetric methods have determined basal concentrations of dopamine to be approximately 1.5 nM (39), compared with 1-3.5 nM estimated with microdialysis measurements (24, 25). Undoubtedly, efforts to minimize tissue damage should be made to ensure accurate results. However, microdialysis has seen widespread use because the data on chemical changes has consistently provided insight into brain functions and pathologies. The efforts to improve temporal resolution for microdialysis will allow it to be used to track chemical changes specific to certain behaviors. Microdialysis may also see more use in the future as a diagnostic tool in human neurosurgery for monitoring neurotrauma (40).
Electrochemical Techniques
Electrochemical detection is one of the most common methods for neurotransmitter monitoring. Many neurotransmitters are electroactive, including dopamine, norepinephrine, epinephrine, and serotonin, and can be detected directly using microelectrodes. The small size of typical microelectrodes, from 7 to 30 μm in diameter, results in less tissue damage than the implantation of larger probes, such as microdialysis probes or conventional electrodes. Three of the most common methods of voltammetric detection for neurotransmitters are amperometry, high-speed chronoamperometry, and fast-scan cyclic voltammetry. In each of these methods, a voltage waveform is applied to the electrode. When the potential is sufficient, electroactive neurochemicals can be oxidized or reduced, and a current is measured that is proportional to the concentration of analyte at the electrode surface. The range of voltages that can be used is finite because of the possible oxidation or reduction of physiological solutions and oxygen, which limit detection to the range of about —1 V to 1.5 V. The shape of the potential waveform applied in each technique is unique, and the resulting chemical information is different for each method (41).
Constant potential amperometry
Amperometry applies a constant potential to the microelectrode that will oxidize the analyte at the electrode surface (Fig. 8a). The current is limited solely by the mass transport rate to the electrode. Measurements can be made with extremely high temporal resolution, typically 500 Hz, because time resolution is not limited by the electrochemistry at the electrode. However, little analyte selectivity occurs with amperometry because a change in the concentration of any molecule electroactive at the applied potential will alter the measured signal.
One major use of amperometry is to measure the efflux of neurotransmitters from individual cells (42). Because the cell type and the molecule being released is known, selectivity is not an issue for these experiments. This technique, developed by R. Mark Wightman, is now widely adopted and involves placing a microelectrode directly above a cell (42). A secretagogue known to stimulate release is applied, and vesicular release is monitored with high temporal resolution. Recently, amperometry has been used to show that ionic secretagogues can influence the exocytotic release of specific neurotransmitters. An example experiment is shown in Fig. 8d, in which dopamine release is monitored after application of K+ to isolated dopaminergic retinal neurons (43). Typical data consists of multiple peaks, each of which is representative of neurotransmitter release from a single vesicle (Fig. 8d). The area under the peak is proportional to the total number of molecules in that vesicle and can be calculated. The amount of dopamine released from retinal cells was calculated to be about 32,000 molecules per vesicle (43). Recent studies have shown that small features detected with amperometry are caused by incomplete vesicular release associated with vesicle fusion (1, 44). Amperometry is a good method for studying basic mechanisms of neurotransmission or the effects of drugs on neurotransmitter release from single cells.

Figure 8. Electrochemical techniques. Applied potential versus time and resulting current versus time graphs for a) amperometry, b) high-speed chronoamperometry, and c) fast-scan cyclic voltammetry. The dotted circle in b) shows where currents are measured after the charging current has decayed. Panels d-f show real data from experiments using each of the techniques. Amperometry data (d) show a current increase that corresponds to detection of electroactive species. Each peak is representative of dopamine release from a single vesicle in retinal neurons, and the area under each peak was used to quantitate the number of molecules released. (Data taken from Ref. (43) by permission from the American Chemical Society.) e) Dopamine concentrations measured by chronoamperometry. Upward-facing arrows indicate time of dopamine injection; downward-facing arrows indicate time of nomifensine injection. Curve A is dopamine detected after injection of 30 pmol of DA in normal tissue, whereas traces B and C are after the dopamine uptake inhibitor nomifensine was administered. Uptake rate is reduced by nomifensine as seen by the increased concentration and the slower return to baseline after injection. (Data from Ref. (47) used by permission from Elsevier.) f) Cyclic voltammetry data of dopamine concentrations from cocaine-seeking rats. The cyclic voltammogram (inset) verifies that dopamine is being detected. The trace shows the current at the dopamine oxidation potential, converted to concentration based on calibration data. The first triangle indicates when the rat approaches the lever, and the second shows when it presses the lever to receive an intravenous injection of cocaine. The dopamine increase before the introduction of cocaine shows that one role of dopamine is as an anticipatory signal for reward. (Data reprinted from Ref. (51) with permission from Macmillan Publishers Ltd.)
Chronoamperometry
For high-speed chronoamperometry, a recurrent square waveform is applied to the electrode, with potentials sufficient enough to oxidize the analyte of interest and subsequently reduce the analyte to the initial form. Rapidly changing the potential causes an inherent charging current at the electrode surface, but this current is dissipated quickly because of the micron size of the electrode. Therefore, the current measured at the end of the step, after the charging current has decayed, is proportional to the analyte concentration (Fig. 8b). The ratio of the peak reduction current to the peak oxidation current is a measure of the reversibility of the electrochemical reaction and can be used to differentiate between some molecules. However, this selectivity is limited, and positively identifying the analyte solely by this technique is difficult. The chronoamperometry waveform is typically repeated at 5-25 Hz.
Greg Gerhardt pioneered a chronoamperometry method that has been used to observe diffusion and uptake parameters in the rat brain (45). In this technique an exogenous neurotransmitter, such as dopamine, is introduced into the brain by pressure ejection or iontophoresis. The microelectrode is a known distance from the ejection pipette, so the analyte concentration detected at the microelectrode is a function of extracellular diffusion and uptake by the dopamine transporter. Because an exogenous neurotransmitter is introduced, the analyte being detected is known, and selectivity is not a problem. After a dopamine ejection, Gerhardt’s group could compare dopamine uptake rates of wild-type rats and rats that had been treated with nomifensine, a dopamine uptake inhibitor (46, 47). They found that nomifensine slowed dopamine uptake rates, as seen by the slower return to baseline in Fig. 8e, and that uptake inhibition increased the concentration of dopamine detected at the electrode. This study was extended to compare dopamine uptake rates with rats that had their transporters disabled during development (47, 48). Recently, chronoamperometry has also been used to show that mice lacking brain-derived neurotrophic factor have slower serotonin clearance caused by impaired serotonin transporter function (49).
Fast-Scan Cyclic Voltammetry
With fast-scan cyclic voltammetry (FSCV), a potential ramp is applied from a reductive holding potential to an oxidative potential and back (Fig. 8c). Scan rates for FSCV are usually between 200 and 1000 V/s, so a scan can be completed in less than 10 ms. A typical repetition rate is 10 Hz, and the electrode is held at the holding potential between scans, which allows for adsorption of the analyte to the electrode. Similar to chronoamperometry, the changing potential at the electrode surface during FSCV causes large charging currents. Because the charging current is relatively stable at carbon electrodes, the current before analyte introduction can be subtracted from the current after analyte introduction. This method is known as background-subtracted FSCV, and it is best used for measuring concentration changes. The data collected is a plot of current versus voltage, called a cyclic voltammogram, which gives a chemical signature of the molecule detected. The peak locations and shapes help differentiate between different molecules, which gives this method the most selectivity of any of the electrochemical techniques discussed here. Some molecules, such as dopamine and norepinephrine, however, have similar CVs and are difficult to resolve.
R. Mark Wightman’s lab used carbon-fiber microelectrodes to pioneer the use of FSCV in measuring dopamine concentration changes with high temporal resolution. Early experiments focused on measuring dopamine concentration changes after electrical stimulation of the dopamine cell bodies (50). In more recent studies, behaviorally evoked dopamine concentrations have been measured. One example is the detection of dopamine in rats when cocaine was self-administered (Fig. 8f) (51). The subsecond temporal resolution allowed the observation that extracellular dopamine increased continuously for about 4 seconds before the lever press for cocaine administration and after the lever press. This observation shows that one role of dopamine is as an anticipatory signal (51). FSCV has also been used to measure other neurotransmitters, such as serotonin (52) and norepinephrine (53), in vivo and in brain slices. Our lab has recently extended the use of FSCV to detect the neuromodulator adenosine successfully. In vivo detection of adenosine and dopamine simultaneously was achieved, and the temporal resolution allowed us to demonstrate that adenosine accumulation was slower than dopamine (38, 54). Fast-scan cyclic voltammetry is the best choice for detecting neurotransmitters that undergo volume transmission in vivo because the cyclic voltammogram provides a means to identify the species detected and the high temporal resolution allows correlation with behavior.
Summary and future directions
The main advantages of electrochemical methods for neurotransmitter detection are the high temporal resolution, the high sensitivity, and the small size of the microelectrode. The different electrochemical techniques have varying levels of selectivity; however, these methods are not as selective as separation methods coupled to microdialysis. The majority of studies have used a combination of anatomical knowledge, pharmacology, and electrochemistry to identify the analyte being detected. Most electrochemical methods are best for measuring fast changes and do not give information about basal levels. A major disadvantage of the electrochemical techniques is that the method is limited to the direct detection of electroactive molecules. New innovations are constantly being made to minimize these limitations. For example, enzyme electrodes are being developed for the indirect detection of nonelectroactive species. Enzymes are immobilized in a polymer coating, and the microelectrode detects an electroactive by-product, such as peroxide, from enzymatic activity (55, 56). Research continues into reducing the size of electrodes and increasing the number of molecules that can be detected simultaneously.
Conclusions and future of the field
Measuring neurochemicals is challenging because the brain is a complex matrix. Most studies have concentrated thus far on determining the effects of a single neurotransmitter. However, future research in this field inevitably will begin to examine interactions between various neurotransmitter systems, and techniques will be needed to measure multiple compounds. Separations-based methods, such as microdialysis, are particularly suited for this challenge. In addition, many researchers are starting to use multiple techniques for a single experiment. An example of this use is the combination of electrochemistry and electrophysiology techniques that can be now employed simultaneously at the same electrode (57). Another challenge is to reduce the amount of damage done to tissue from invasive techniques. Methods to microfabricate microdialysis probes or reduce the size of microelectrodes would be useful for maintaining tissue integrity. Noninvasive techniques, such as imaging, are becoming more prominent, especially for human studies. Many of the most popular methods, such as functional magnetic resonance imaging, measure changes in oxygen levels and blood flow but do not directly measure neurotransmitters (58). Positron emission tomography measures changes in receptor occupancy, a more direct measure of neurotransmission (59), but advances are needed to synthesize more positron-emitting ligands for different receptors. Imaging methods for cellular activity are also becoming more popular. For example, fluorescence resonance energy transfer (FRET) can be used to detect binding at receptors (60). However, these studies remain fairly complicated and often require genetic engineering of FRET-based pairs into a system. Optical probes, such as quantum dots or fluorescence-based dyes, may also soon see widespread use in detecting chemical changes in cell culture or brain slices.
The biggest advances for in vivo experiments will be in the area of designing better techniques for behaving animals. For electrochemistry, several groups have explored a wireless system of data collection in which the controls for electrochemistry and data are collected in instrumentation that can be placed in a backpack on the rat (61). The data then is sent wirelessly to a computer, so that the animal is not tethered to the computer. Similarly, telemetric electrophysiological systems are being developed that will enable wireless collection of unit recordings (62). These systems can be used to record spikes of neuronal activity in small behaving animals, such as birds. Integrated systems that measure both behavioral activity and neurochemical changes will also continue to be developed. Making measurements of neurotransmitters will continue to be challenging, but new techniques will lead to better insight into the basic neurobiology and regulation of neurotransmission.
References
1. Staal RG, Mosharov EV, Sulzer D. Dopamine neurons release transmitter via a flickering fusion pore. Nat. Neurosci. 2004; 7:341-346.
2. Smith CUM. Elements of Molecular Neurobiology, 2nd edition. 1996. Wiley, New York.
3. Zoli M, Torri C, Ferrari R, Jansson A, Zini I, Fuxe K, Agnati LF. The emergence of the volume transmission concept. Brain Res. Rev. 1998; 26:136-147.
4. Verma S, Robertson AP, Martin RJ. The nematode neuropeptide, AF2 (KHEYLRF-NH2), increases voltage-activated calcium currents in Ascaris suum muscle. Br. J. Pharmacol. 2007; 151:888-899.
5. Gerevich Z, Wirkner K, Illes P. Adenosine A(2A) receptors inhibit the N-methyl-D-aspartate component of excitatory synaptic currents in rat striatal neurons. Eur. J. Pharmacol. 2002; 451:161-164.
6. Takahashi A, Tokunaga A, Yamanaka H, Mashimo T, Noguchi K, Uchida I. Two types of GABAergic miniature inhibitory postsynaptic currents in mouse substantia gelatinosa neurons. Eur. J. Pharmacol. 2006; 553:120-128.
7. Jardemark K, Orwar O, Jacobson I, Moscho A, Zare RN. Patch clamp detection in capillary electrophoresis. Anal. Chem. 1997; 69:3427-3434.
8. Eghbali M, Curmi JP, Birnir B, Gage PW. Hippocampal GABA(A) channel conductance increased ky diazepam. Nature. 1997; 388:71-75.
9. Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, Oh U. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc. Natl. Acad. Sci. USA. 2000; 97:6155-6160.
10. Graham DM, Wong KY, Shapiro P, Frederick C, Pattabiraman K, Berson DM. Melanopsin ganglion cells use a membrane-associated rhabdomeric phototransduction cascade. J. Neurophys. 2008; 99:2522-2532.
11. Song D, Chan RHM, Marmarelis VZ, Hampson RE, Deadwyler SA, Berger TW. Nonlinear dynamic modeling of spike train transformations for hippocampal-cortical prostheses. IEEE. T. Bio-med. Eng. 2007; 54:1053-1066.
12. Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007; 30:203-210.
13. Pereira A, Ribeiro S, Wiest M, Moore LC, Pantoja J, Lin SC, Nicolelis MA. Processing of tactile information by the hippocampus. Proc. Natl. Acad. Sci. U.S.A. 2007; 104:18286-18291.
14. Christova L, Stephanova D, Kossev A. Branched EMG electrodes for stable and selective recording of single motor unit potentials in humans. Biomedizinische Technik. 2007; 52:117-121.
15. Landolt HP. Sleep homeostasis: A role for adenosine in humans? Biochem Pharmacol. 2008; 75:2070-2079.
16. Hamill OP, McBride DW. Induced membrane hypo/hypermechanosensitivity: A limitation of patch-clamp recording. Annu. Rev. Physiol. 1997; 59:621-631.
17. Fertig N, Blick RH, Behrends JC. Whole cell patch clamp recording performed on a planar glass chip. Biophys. J. 2002; 82:3056- 3062.
18. Priest BT, Swensen AM, McManus OB. Automated electrophysiology in drug discovery. Curr. Pharm. Design 2007; 13:2325-2337.
19. Semmler JG, Kornatz KW, Dinenno DV, Zhou S, Enoka RM. Motor unit synchronisation is enhanced during slow lengthening contractions of a hand muscle. J. Phys. 2002; 545:681-695.
20. Brown EN, Kass RE, Mitra PP. Multiple neural spike train data analysis: state-of-the-art and future challenges. Nat. Neurosci. 2004; 7:456-461.
21. Chaurasia CS. In vivo microdialysis sampling: theory and applications. Biomed. Chromatogr. 1999; 13:317-332.
22. Jing FC, Chen H, Li CL. Rapid determination of dopamine and its metabolites during in vivo cerebral microdialysis by routine high performance liquid chromatography with electrochemical detection. Biomed. Envi. Sci. 2007; 20:317-320.
23. Bungay PM, Newton-Vinson P, Isele W, Garris PA, Justice JB. Microdialysis of dopamine interpreted with quantitative model incorporating probe implantation trauma. J. Neurochem. 2003; 86:932-946.
24. Tang A, Bungay PM, Gonzales RA. Characterization of probe and tissue factors that influence interpretation of quantitative microdialysis experiments for dopamine. J. Neurosci. Methods 2003; 126:1-11.
25. Parsons LH, Smith AD, Justice J. The in vivo microdialysis recovery of dopamine is altered independently of basal level by 6-hydroxydopamine lesions to the nucleus accumbens. J. Neurosci. Meth. 1991; 40:139-147.
26. Cui YL, Zhang L, Utsunomiya K, Yanase H, Mitani A, Kataoka K. Ischemia-induced glutamate release in the dentate gyrus. A microdialysis study in the gerbil. Neurosci. Lett. 1999; 271:191-194.
27. Benveniste H, Drejer J, Schousboe A, Diemer NH. Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral-ischemia monitored by intracerebral microdialysis. J. Neurochem. 1984; 43:1369-1374.
28. Jellish WS, Zhang X, Langen KE, Spector MS, Scalfani MT, White FA. Intrathecal magnesium sulfate administration at the time of experimental ischemia improves neurological functioning by reducing acute and delayed loss of motor neurons in the spinal cord. Anesthesiology. 2008; 108:78-86.
29. Cadoni C, Di Chiara G. Differences in dopamine responsiveness to drugs of abuse in the nucleus accurnbens shell and core of Lewis and Fischer 344 rats. J. Neurochem. 2007; 103:487-499.
30. Chefer VI, Zakharova I, Shippenberg TS. Enhanced responsiveness to novelty and cocaine is associated with decreased basal dopamine uptake and release in the nucleus accumbens: Quantitative microdialysis in rats under transient conditions. J. Neurosci. 2003; 23:3076-3084.
31. Venton BJ, Robinson TE, Kennedy RT. Transient changes in nucleus accumbens amino acid concentrations correlate with individual responsivity to the predator fox odor 2,5-dihydro-2,4,5-trimethylthiazoline. J. Neurochem. 2006; 96:236-246.
32. Hotsenpiller G, Wolf ME. Baclofen attenuates conditioned locomotion to cues associated with cocaine administration and stabilized extracellular glutamate levels in the rat nucleus accumbens. Neurosci. 2003; 118:123-134.
33. Venton BJ, Robinson TE, Kennedy RT, Maren S. Dynamic amino acid increases in the basolateral amygdala during acquisition and expression of conditioned fear. Eur. J. Neurosci. 2006; 23:3391-3398.
34. Lena I, Parrot S, Deschaux O, Muffat-Joly S, Sauvinet V, Renaud B, Suaud-Chagny MF, Gottesmann C. Variations in extracellular levels of dopamine, noradrenaline, glutamate, and aspartate across the sleep-wake cycle in the medial prefrontal cortex and nucleus accumbens of freely moving rats. J. Neurosci. Res. 2005; 81:891-899.
35. Cellar NA, Burns ST, Meiners JC, Chen H, Kennedy RT. Microfluidic chip for low-flow push-pull perfusion sampling in vivo with on-line analysis of amino acids. Anal. Chem. 2005; 77:7067-7073.
36. Khan AS, Michael AC. Invasive consequences of using microelectrodes and microdialysis probes in the brain. TrAC. Trends Anal. Chem. 2003; 22:503-508.
37. Clapp-Lilly KL, Roberts RC, Duffy LK, Irons KP, Hu Y, Drew KL. An ultrastructural analysis of tissue surrounding a microdialysis probe. J. Neurosci. Methods. 1999; 90:129-142.
38. Swamy BEK, Venton BJ. Susbsecond detection of physiological adenosine concentrations using fast-scan cyclic voltammetry. Anal. Chem. 2007; 79:744-750.
39. Crespi F, Mobius C. In vivo selective monitoring of basal levels of cerebral dopamine using voltammetry with Nafion modified (NA-CRO) carbon fibre microelectrodes. J. Neurosci. Methods 1992; 42:149-161.
40. Parkin MC, Hopwood SE, Jones DA, Hashemi P, Landolt H, Fabricius M, Lauritzen M, Boutelle MG, Strong AJ. Dynamic changes in brain glucose and lactate in pericontusional areas of the human cerebral cortex, monitored with rapid sampling on-line microdialysis: relationship with depolarisation-like events. J. Cereb. Blood Flow. 2005; 25:402-413.
41. Michael DJ, Wightman RM. Electrochemical monitoring of biogenic amine neurotransmission in real time. J. Pharm. Biomed. Anal. 1999; 19:33-46.
42. Leszczyszyn DJ, Jankowski JA, Viveros OH, Diliberto EJ Jr., Near JA, Wightman RM. Nicotinic receptor-mediated catecholamine secretion from individual chromaffin cells. Chemical evidence for exocytosis. J. Biol. Chem. 1990; 265:14736-14737.
43. Hochstetler SE, Puopolo M, Gustincich S, Raviola E, Wightman RM. Real-time amperometric measurements of zeptomole quantities of dopamine released from neurons. Anal. Chem. 2000; 72:489-496.
44. Wightman RM, Haynes CL. Synaptic vesicles really do kiss and run. Nat. Neurosci. 2004; 7:321-322.
45. Zahniser NR, Dickinson SD, Gerhardt GA. High-speed chronoamperometric electrochemical measurements of dopamine clearance. Methods Enzymol. 1998; 296:708-719.
46. Costall B, Kelly DM, Naylor RJ. Nomifensine: a potent dopaminergic agonist of antiparkinson potential. Psychopharmacologia 1975; 41:153-164.
47. Luthman J, Friedemann MN, Hoffer BJ, Gerhardt GA. In vivo electrochemical measurements of exogenous dopamine clearance in normal and neonatal 6-hydroxydopamine-treated rat striatum.Exp. Neurol. 1993; 122:273-282.
48. Cass WA, Zahniser NR, Flach KA, Gerhardt GA. Clearance of exogenous dopamine in rat dorsal striatum and nucleus accumbens: role of metabolism and effects of locally applied uptake inhibitors. J. Neurochem. 1993; 61:2269-2278.
49. Daws LC, Munn JL, Valdez MF, Frosto-Burke T, Hensler JG. Serotonin transporter function, but not expression, is dependent on brain-derived neurotrophic factor (BDNF): in vivo studies in BDNF-deficient mice. J. Neurochem. 2007; 101:641-651.
50. Robinson DL, Venton BJ, Heien MLAV, Wightman RM. Detecting subsecond dopamine release with fast-scan cyclic voltammetry in vivo. Clin. Chem. 2003; 49:1763-1773.
51. Phillips PEM, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release triggers cocaine seeking. Nature. 2003; 422:614-618.
52. Bunin MA, Wightman RM. Quantitative evaluation of 5-hydroxytryptamine (serotonin) neuronal release and uptake: an investigation of extrasynaptic transmission. J. Neurosci. 1998; 18:4854- 4860.
53. Miles PR, Mundorf ML, Wightman RM. Release and uptake of catecholamines in the bed nucleus of the stria terminalis measured in the mouse brain slice. Synapse. 2002; 44:188-197.
54. Cechova S, Venton BJ. Transient adenosine efflux in the rat caudate-putamen. J. Neurochem. 2008; 105:1253-1263.
55. Kulagina NV, Michael AC. Monitoring hydrogen peroxide in the extracellular space of the brain with amperometric microsensors. Anal. Chem. 2003; 75:4875-4881.
56. Oldenziel WH, Dijkstra G, Cremers TIFH, Westerink BHC. In vivo monitoring of extracellular glutamate in the brain with a microsensor. Brain Res. 2006; 1118:34-42.
57. Cheer JF, Aragona BJ, Heien MLAV, Seipel AT, Carelli RM, Wightman RM. Coordinated accumbal dopamine release and neural activity drive goal-directed behavior. Neuron. 2007; 54:237-244.
58. Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001; 412:150-157.
59. Pappata S, Salvatore E, Postiglione A. In vivo imaging of neurotransmission and brain receptors in dementia. J. Neuroimaging. 2008; 18:111-124.
60. Lohse MJ, Bunemann M, Hoffmann C, Vilardaga JP, Nikolaev VO. Monitoring receptor signaling by intramolecular FRET. Curr. Opin. Pharmacol. 2007; 7:547-553.
61. Garris PA, Ensman R, Poehlman J, Alexander A, Langley PE, Sandberg SG, Greco PG, Wightman RM, Rebec GV. Wireless transmission of fast-scan cyclic voltammetry at a carbon-fiber microelectrode: proof of principle. J. Neurosci. Methods. 2004; 140:103-115.
62. Schregardus DS, Pieneman AW, Ter Maat A, Jansen RF, Brouwer TJF, Gahr ML. A lightweight telemetry system for recording neuronal activity in freely behaving small animals. J. Neurosci. Methods. 2006; 155:62-71.
Further Reading
Zigmond MJ, Bloom FE, Landis SC, Roberts JL, Squire LR. Fundamental Neuroscience. 1999. Academic Press, New York.
Johnston D, Wu M. Foundations of Cellular Neurophysiology. 1995. Bradford Books, London, UK.
Joukhadar C, Muller M. Microdialysis: Current Applications in Clinical Pharmokinetic Studies and its Potential Role in the Future. Clinical Pharmacokinetics 2005; 44:895-913.
Michael AC, Borland LM. Electrochemical Methods for Neuroscience. 2007. CRC Press, New York.