CHEMICAL BIOLOGY
Pharmaceuticals: Natural Products and Natural Product Models
Sheo B. Singh, Merck Research Laboratories, Rahway, New Jersey
doi: 10.1002/9780470048672.wecb444
Natural products have played a vital role in the treatment of human ailments for thousands of years and continue to play a big role in the modern discovery of new agents for the treatment of diseases today. In certain therapeutic areas, natural products account for almost all key modern medicine used today. Many drugs are formulated and used directly as they are found in nature, some are derived directly from natural products by semisynthesis, and others are modeled after natural products. In this overview, examples of the pharmaceutically important natural products have been summarized.
Introduction
Natural product preparations have played a vital role in empiric treatment of ailments for thousands of years in many advanced civilizations and continue to play a significant role even today in various parts of the world. The use of plants and preparations derived from plants has been the basis for the sophisticated medical treatments in Chinese, Indian, and Egyptian civilizations for many thousands of years. These medical applications have been documented in the Chinese Materia Medica (1100 BC), in the Indian Ayurveda (1000 BC), and in Egyptian medicine as early as 2900 BC. Plant preparations continued to be the basis of medical treatments in the ancient Western world as well. This knowledge migrated through Greece to Western Europe, including England, during the ancient period and led to its formal codification in the United Kingdom and to the publication of the London Pharmacopoeia in 1618.
The isolation of strychnine (1), morphine (2), atropine (3), colchicine (4), and quinine (5) in the early 1800s from the commonly used plants and their use for the treatment of certain ailments might constitute the early idea of “pure” compounds as drugs. E. Merck isolated and commercialized morphine (2) as the first pure natural product for the treatment of pain (1-3). Preparations of the Willow tree have been used as a painkiller for a long period in traditional medicine. Isolation of salicylic acid (6) as the active component followed by acetylation produced the semisynthetic product called “Aspirin” (7) that was commercialized by Bayer in 1899 for the treatment of arthritis and pain (4).
The World Health Organization estimates that herbal and traditional medicines, derived mostly from plants, constitute primary health care for ~80% of the world population even today. The compounds produced by plants play significant roles in the treatment of diseases for the rest of the 20% of populations that are fortunate to use modern medicine. About 50% of the most prescribed drugs in the United States consist of natural products or their semisynthetic derivatives, or they were modeled after natural products. “Curare,” the crude extract from the South American plant, Chondodendron tomentosum, and the derived purified compound tubocurarine, has been used as anesthetic in surgery until recently. Purified digitoxin, as well as the crude extracts that contain digitalis glycosides from foxglove plant, Digitalis lanata, is used as cardiotonic even today.
Drugs derived from microbial fermentations have played perhaps a bigger role in the modern drug discovery and have revolutionized the practice of medicine, which leads to saving human lives. Although the contribution of purified natural products as single agent drugs is significant in almost all therapies, their contribution in the treatment of bacterial infection is perhaps most critical (5). Natural products constitute drugs or leads to all but three classes of antibiotics. The discovery of microbial natural products-based antibiotics began with the serendipitous observation by Fleming in 1929 that bacterial growth was prevented by the growth of Penicillium notatum. Although this discovery was highly publicized and very important, it took over 10 years before the active material, penicillin, was purified and structurally elucidated by Florey and Chain in early 1940s. Subsequent commercialization was very quick, driven largely by the medical needs of World War II. Penicillin was one of the first broad-spectrum antibiotics that treated bacterial infection and saved millions of lives. Fleming, Florey, and Chain were awarded the Nobel Prize in 1945 for their efforts on penicillin. The success of penicillin led to unparalleled efforts by government, academia, and the pharmaceutical industry to focus drug-discovery efforts based on the newfound “microbial” sources for the discovery of natural products beyond plants. However, initial efforts were mostly focused on the discovery of antibiotic compounds from fermentations of a variety of microorganisms of not only fungal origin by also soil-dwelling prokaryotes (e.g., Streptomyces spp.), which led to the discovery by 1962 of almost all novel classes of antibiotic scaffolds that are being used today. The antibiotic discovery effort was performed largely by Fleming’s method of detection of antibacterial activity on petri plates. Zones of inhibition of bacterial strains on agar plates were measured after applying whole broth or extracts obtained from microbial ferments (5). As newer biological assays and screening techniques became available in the 1960s, microbial sources, along with plant and marine sources, started to be used for screening against other therapeutic targets, which led to the discovery of leads and drugs in those areas. Examples of these discoveries will be discussed with the target areas. As time progressed, improved technologies in biology and chemistry helped with the popularization of natural products; natural product extracts became part of the screening resource in most large pharmaceutical houses from 1960 through the 1980s until their de-emphasis in the early 1990s. Therefore, natural product extracts became popular sources for the screening against purified enzymes and receptors, an occurrence that led to the identification of many nonantibiotic natural products that have revolutionized the practice of medicine, saved countless human lives, improved quality of life, and perhaps helped increase life expectancy for humans.

Antibacterial Agents
Natural products contribute to over 80% of all antibiotics that are in clinical practice today. Natural products contribute to all but three classes of antibiotics (3).
Penicillin was the first β-lactam and the first broad-spectrum antibiotic discovered that started the “Golden age” (1940-1962) of antibiotics. The structure of penicillin contains a thiazolidine ring that is fused to a β-lactam ring. The existence and stability of the β-lactam ring was highly controversial at the time despite the availability of a single crystal X-ray structure of one of the penicillins. Penicillin G (8) was the first penicillin that was clinically used. Penicillin G was converted easily by either chemical or biochemical means to 6-amino-penicillanic acid (9), which became the lead for the semisynthetic modifications that led to the synthesis of various penicillin derivatives. Some early derivatives (e.g., amoxicillin 10) are still in clinical use. Penicillins (general structure 11) bind to penicillin-binding proteins and inhibit the bacterial cell wall. Penicillins became targets of β-lactamases that opened the β-lactam ring and abolished the antibacterial effectiveness of these compounds (5, 6).
Cephalosporin C (12), a second class of β-lactam antibiotics, was first discovered from Cephalosporium acremonium, isolated from a sewer outfall of Sardinia, Italy in 1948. Although Cephalosporin C was less active than penicillin G, it was less prone to β-lactamase action and therefore attracted a lot of attention that led to the development of five generations of orally active clinical agents (e.g., cephalexin, 13 and general structure, 14) (5, 6).
Continued search for even better antibiotics led to the discovery of the highly potent and broadest-spectrum antibiotic thienamycin (15), the third class of the P-lactams, called carbapenems, in which the sulfur atom of the thiazolidine ring was replaced by a methylene group. Thienamycin was produced by Streptomyces cattleya (7). The primary amine group of thienamycin self-catalyzes the opening of the β-lactam ring, which leads to the concentration-dependent instability that poses a serious challenge for the fermentation-based production of the compound. The Merck group stabilized the compound by replacing the primary amine with an aminomethylidineamino group and synthesized imipenem (16) (8). They developed a highly efficient total synthesis that remains in commercial use today. Imipenem was approved for clinical use 23 years ago in 1985, but it remains one of the most important broad-spectrum hospital antibiotics in the market today. Like other β-lactams, several generations of carbapenems (general structure 17) have been approved for clinical use in recent years (9).
As resistance to β-lactam antibiotics increased because of the expression of a variety of β-lactamases, many groups focused their efforts on discovering compounds that could be more reactive to β-lactamases without having significant intrinsic antibiotic activities of their own and pharmacokinetic properties that would be similar to β-lactam antibiotics. This focus led to the discoveries of clavulanic acid (18) and monobactam sulfazecins (19). Nature effectively stabilized the latter monobactam structure by the addition of a N-sulfamic acid. The β-lactamase inhibitor clavulanic acid was combined with amoxicillin, which led to the development of a potent and successful antibacterial agent, Augmentin® (GSK, Surrey, UK) (10). Chemical modifications of the monobactam produced aztreonam (20), a clinical agent with a narrow spectrum but significantly improved activity against Gram-negative pathogens, particularly Pseudomonas aeriginosa (11).

Immediately after the discovery of penicillin, Waksman started efforts on soil-dwelling bacteria and discovered the first of the aminoglycosides, streptomycin (21) from Streptomyces griseus, in 1943 (6). Subsequently, a series of aminoglycosides was isolated. These aminoglycosides are potent broad-spectrum antibiotics and are potent inhibitors of protein synthesis. Unfortunately, nephrotoxicity limited their wider use, and they are used mainly for treatment of infections caused by Gram-negative bacteria. Continued efforts to screen prokaryotic organisms led to the discovery of the phenyl propanoids (chloroamphenicol, 22) and tetracyclines. The latter is a major class of tetracyclic polyketides that were discovered from various species of Streptomyces spp. Although the parent tetracycline (23) was not used as an antibiotic to a great extent, the chloro derivative (Clortetracycline 24), oxytetracycline (25), and minocycline (26) are clinical agents. This class of compound suffered from the selection for rapid resistance via efflux mechanism that limited their use (6). Recently, however, chemical modifications of the A-ring yielded compounds that overcame the efflux pump and lead to the development of tigecycline (27) as an effective broad spectrum antibiotic (12).
Another large class of orally active protein syntheis inhibitor antibiotics that were produced by Streptomyces spp. is represented by 14-membered lactones generically callsed macrolides, exemplified by the first member, erythromycin (28) (6). Chemical modifications of this class of compounds led to many clinical agents such as the aza derivatives, azithromycin (29) and ketolide (telithromycin, 30) (13, 14). Mupirocin (pseudomonic acid, 31) is another protein synthesis inhibitor that was isolated from Pseudomonas fluorescens and is used only as a topical agent (6).

Vancomycin (32), a glycopeptide produced by Streptomyces orientalis, is a key Gram-positive antibiotic, originally discovered in 1954, and remains a critical antibiotic in clinical practice even today for the treatment of Gram-positive bacterial infections (6). Teicoplanin (33), a related glycopeptide produced by Streptomyces teicomyceticus, is a newer antibiotic that complements vancomycin in the clinic but is not effective against vancomycin-resistant bacteria. Ramoplanin (34) represents another glycopeptide that is larger in molecular size and structurally different from vancomycin and teicoplanin; it is in the late stages of clinical development for treatment of Gram-positive bacterial infections. Glycopeptides inhibit the bacterial cell wall. Daptomycin (35), a cyclic lipopeptide produced by Streptomyces roseosporus, is one of the newest members of antibiotics approved for the clinical practice as a broad-spectrum Gram-positive agent. It works by depolariztion of the bacterial cell membrane (14). Streptogramins were discovered in the early 1960s but were used for humans only recently when a 70/30 mixture of dalfopristin (36) and quinupristin (37) with the trade name Synercid® King Pharmaceuticals, Bristol, NJ; was developed for the treatment of drug-resistant Gram-positive bacterial infections (15).

Antibiotics from natural sources range from compounds with small molecular size (e.g., thienamycin) to large peptides (e.g., ramoplanin). They generally possess complex architectural scaffolds and densely deployed functional groups, which affords the maximal number of interactions with molecular targets and often leads to exquisite selectivity for killing pathogens versus the host. This function is nicely illustrated by vancomycin binding to its target. Vancomycin has five hydrogen bond contacts with the D-Ala-D-Ala terminal end of peptidoglycan. Resistant organisms modify the terminal D-Ala with D-lactate, which leads to loss of one hydrogen bond and a 1000-fold drop in binding affinity and loss of antibiotic activity (see Fig. 1) (6).
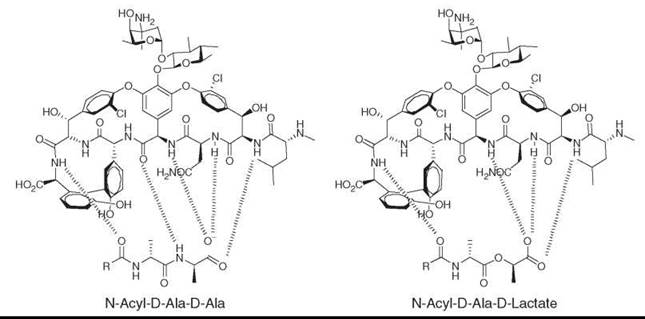
Figure 1. Vancomycin binding to the active site D-Ala-D-Ala of peptidoglycan (left panel) and D-Ala-D-Lactate (right panel).
Antifungal Agents
Significant similarities in the fungal and mammalian cellular processes result in very few fungal-specific drug targets that lead to the development of only a few quality and safe antifungal agents. Amphotericin B (38), a natural product that consists of a polyene lactone, is a highly effective broad-spectrum antifungal agent unfortunately with a very limited safety margin. Recently, glucan synthesis was identified as a fungal-specific target that could be inhibited by a series of cyclic peptides called echinocandins, which were identified in the 1970s as having potent antifungal activities (16, 17). This identification provided impetus for the discovery and development of new related lipopeptides that led to the identification of pneumocandins from Glared lozoyensis (e.g., pneumocandin Bo, 39). Chemical modifications at two sites of pneumocandin Bo led to the synthesis of caspofungin (40), which was the first in the class of glucan synthesis inhbitors; it is a “potent”, highly effective, and safe antifungal agent approved for serious fungal infections in hospitals. Side chain replacements of the related cyclic peptide FR901379 (isolated from the fungus Coleophoma empetri) and echinocandin B (isolated from Aspergillus nidulans) led to two additional clinical agents of this class, micafungin (41) and anidulafungin (42), respectively. (16, 17).

Antimalarials
Quinine (5) isolated from Cinchona bark was one of the first antimalarials discovered, and it became a model for the discovery and development of some of the most successful antimalarial agents, chloroquine and its successors. However, the development of resistance by the malarial parasite Plasmodium falciparum for these drugs has rendered them ineffective. Artemisinin (43), a sesquiterpene peroxide originally isolated from a Chinese herb Artemisia annua in 1972 as an antimalarial agent, was chemically modified to a derivative, artemether (44), which is a very effective and widely used antimalarial agent (18). Unfortunately, limited supply of this plant-derived compound rendered it inaccessible for wider use. Recently, biosynthetic genes of artimisinin have been identified and successfully transfected to an heterologous host, Escherichia coli. This method has allowed the production of an intermediate, amorphadiene (45) and artemisinic acid (46), which could be transformed chemically to artmether and potentially could relieve the strain of supply and could provide wider availability (19-21).
Antivirals
Most antiviral agents are based on nucleoside structures and have their origin from spongouridine (47) and spongothymidine (48) that were isolated from marine sponges in the 1950s by Bergmann and his coworkers (22-24). These natural nucleosides possessed sugars other than ribose and deoxyribose and provided rationale for the substitution of the sugars in the antiviral nucleosides with various sugar mimics, including linear polar groups that led to the synthesis of Ara-A (49) and acyclovir (50). HIV protease inhibitors were developed from pepstatin (51), a pepsin inhibitor produced by various fungal species. Pepstatin possesses as the structural component statine a β-hydroxy-γ-amino acid that mimics the transition-state intermediate of the hydrolytic reaction catalyzed by the proteases (25). This structure became the foundation for the rational peptidomimetics and design of all HIV protease inhibitors, for example, indinavir (Crixivan®, Merck & Co., Inc., Whitehouse Station, NJ; 52) and others (26).

Antipain Agents
Use of the opium poppy (Papaver somniferum) to ameliorate pain dates back thousands of years, and the active metabolite morphine (2) was isolated first from its extracts in 1806 followed by codeine (53) in 1832 (27, 28). Morphine and its derivatives are agonists of opiate receptors in the central nervous system and are some of the most effective pain relievers known and prescribed for postoperative pain. Morphine and codeine differ by substitution by methyl ether. Unfortunately, addictive properties of these compounds limit their use. Efforts have been made to reduce the addictive properties of morphine, which resulted in a semisynthetic derivative buprenorphine (54) (29). This compound is 25 to 50 times more potent than morphine with lower addictive potential and has been indicated for use by morphine addicts.

Conotoxins, a class of 10 to 35 amino acid-containing peptides produced by cone snails to intoxicate their prey, were isolated and characterized by Olivera and coworkers (30). They are a novel class of analgesics that helped identify the target and blocking of N-type Ca+2 channels. One compound, Ziconotide (55), was synthesized and developed as a treatment for severe chronic pain (31, 32).
The alkaloid epibatidine (56) was discovered from the skin of an Ecuadorian poison frog (Epipedobates tricolor), and its potent analgesic activity was demonstrated as early as in 1974 by Daly and coworkers (33-35). The paucity of the material delayed the structure elucidation and was only accomplished after the invention of newer and more sensitive NMR techniques in 1980s. Once the structure was elucidated as chloronicotine derivative, it was synthesized and was shown to antagonize nicotinic receptors in neurons. It did not show any specificity with similar receptors in other tissues and lacked a therapeutic index of any clinical value. However it served as a model for designing compounds with desired specificity and resulted in the synthesis of ABT594 (57), which is an agonist of the nicotinic acetylcholine receptor, is about 50-fold more potent than morphine without addictive properties, and was under advanced clinical development until its discontinuance in 2003 (36-39).

Antiobesity
Lipstatin (58), comprised of a 3,5-dihydroxy-2-hexyl hexadeca-7,9-dienoic acid with cyclization of the hydroxy group at C-3 with the carboxylic acid to form a β-lactone and esterification of the hydroxy group at C-5 with a N-formyl leucine, was isolated from Streptomyces toxytricini. It inhibits gastric and pancreatic lipase and blocks intestinal absorption of lipids (40, 41). Reduction of the olefins led to the synthesis of tetrahydrolipstatin, which was approved in 1999 as Xenical (59) for the treatment of obesity (42-44).
Alzheimer's Agents
Galantamine (60) is a tetracyclic alkaloid that was isolated originally from Galanthus nivalis and subsequently from Narcissus spp. (45). It was approved by trade name Reminyl® Johnson & Johnson, New Brunswick, NJ for the treatment of Alzheimer’s disease. That galantamine, a selective inhibitor of acetylcholinesterase, was confirmed by X-ray structural characterization of galantamine bound to a plant acetylcholinesterase (46, 47).
Antineoplastic Agents
Natural products have played a much bigger role, perhaps second only to antibacterial agents, in the discovery and development of anticancer agents either directly as drugs or leads to drugs (48). In fact, they contribute 64% of all approved cancer drugs (3). Taxol® BMS, NY (61, paclitaxel) is unarguably the most successful anticancer drug in clinical use. It is a taxane diterpenoid that was isolated from the Pacific yew Taxus brevifolia in 1967 as a cytotoxic agent but not pursued as a development candidate until its novel mode of action was determined in 1979 as a stabilizer of microtubule assembly (49-52). The discovery of the mechanism of action led to the United States National Cancer Institute (NCI) committing significant resources to the large-scale production, eventual clinical development, and approval of Taxol® (paclitaxel) by U.S. Food and Drug Administration in 1992 for treatment of breast, lung, and ovarian cancer. Another important plant product, camptothecin (62), an alkaloid from Camptotheca acuminata, was discovered in 1966, also by the Wall and Wani group (53). The development of this compound was also hampered by the lack of knowledge of the mechanism of action and most importantly by its extremely poor water solubility. Determination of the mechanism of action as an inhibitor of topoisomerases-I led to significant efforts both by NCI and the pharmaceutical industry, which resulted in chemical modification of the structure, introduction of water-solubilizing groups (e.g., amino), and development of several derivatives as anticancer agents exemplified by topotecan (63) and irinotecan (64) (54, 55).
The Vinca alkaloids, vinblastine (64) and vincristine (65), isolated from Catharanthus roseus, have contributed significantly to the understanding and treatment of cancer (56, 57). These compounds bind to tubulin and inhibit cell division by inhibiting mitosis; they were perhaps the best-known anticancer agents before Taxol®. Chemical modifications of vinblastine led to the clinical agents vinorelbine (66) (58) and vindesine (67) (48 one, 59-61). Podophyllotoxin (68) was isolated from various species of the genus Podophyllum spp. as an anticancer agent. Chemical modifications of the naturally occurring epimer, epipodophyllotoxin (69), led to the synthesis and development of etoposide (70) and teniposide (71) as clinical agents (48, 54, 62-65).
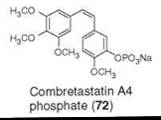
Combretastatin A4 phosphate (72) is a phosphate prodrug of combretastatin A4, a cis-stilbene, isolated from Combretum caffrum (66). Combretastatin A4 is one of the many combretastatins that inhibits tubulin polymerization (67), shows efficacy against solid tumor, is a vascular targeting agent that blocks the blood supply to solid tumors, and is in Phase II/III clinical development for the treatment of various types of tumors as a vascular targeting agent (68-71).

Microbial sources have been a very rich source for cancer chemotherapeutic agents. Of particular note is the Streptomyces spp., which has been responsible for the production of many approved anticancer agents that are in clinical practice. These agents are represented by highly diverse structural classes exemplified by the anthracycline family (e.g., doxorubicin, 73) (72-74), actinomycin family (e.g., dactinomycin, 74), glycopeptides family (e.g., bleomycins A2 and B2, 75 and 76) (75), and mitomycin family (e.g., mitomycin C, 77) (72, 76). All these compounds specifically interact with DNA for their mode of action.

Staurosporine (78) produced by Streptomyces spp. is a potent inhibitor of protein kinase C (77-79). This compound inhibits many other kinases with almost equal potency and has become a great tool for the study of kinases. Lack of selectivity for protein kinase C has significantly hampered the development of this compound. Recently, however, several compounds derived from this lead have entered in the clinic for potential treatment of cancer. These include 7-deoxystaurosporine (79) and CGP41251 (80) (80, 81). CGP41251 shows multiple modes of action including inhibition of angiogenesis in vivo.
Microbial sources other than Streptomyces spp. have also provided highly interesting and structurally diverse compounds. Discovery of epothilones from myxobacterial strains by a German group (82) and the Merck group (83, 84) constitute a breakthrough discovery. The Merck group used an assay that mimicked Taxol® at the active site for the screening of natural products that led to the isolation of epothilones A (81) and B (82). The discovery of a unique structural class, interesting biological activity, and clinically proven mode of action drew significant attention from the scientific community and led to a variety of approaches, including combinatorial biosynthesis, chemical modifications, and total synthesis, that permitted preparation of many derivatives with improved potency and drug-like properties. A series of these compounds have entered human clinical trials, and many are in the late stages of development. Epothilone discovery and development has been recently reviewed (85).

Recent pursuit of marine microbial sources led to the isolation of salinosporamide A (83). It is a β-lactone produced by the marine bacteria Salinispora tropica and is a proteasome inhibitor (86). Mechanistically, it works by specific covalent modification of the target. This compound has entered human clinical development for treatment of multiple myeloma (87-89).
Although use of marine microbial sources for the discovery of natural products is a somewhat recent phenomenon, marine natural products from higher species have contributed tremendously to the discovery of novel architecturally complex compounds as anticancer agent leads with one, Ecteinascidin-743, now approved in the European Union for treatment of sarcoma. The discovery of natural products derived from marine sources exploded in the 1970s not only because of increased level of NCI funding but also because of technological advancements in the techniques for collection of specimens, chemical isolation, and structural elucidation of low amounts of compounds initially isolated. Because of the fear of a limited supply of marine sources for large-scale production, marine natural products have remained the exclusive purview of academia except for a small Spanish pharmaceutical company, PharmaMar, which collaborates closely with academia and governments. Bryostatins are among the most interesting marine natural products known. They were isolated from the bryozoan Bugula neritina. They are a series of polyketide macro lactones represented here by the major congener bryostatin I (84), which is a modulator of protein kinase C and has been subjected to several human clinical trials (90, 91). Recently, combination studies have been recommended for Phase I and Phase II trials, mainly under the auspices of the NCI. Modeling studies along with a diligent chemical design approach has led to the synthesis of a simplified analog 85 that has been shown to be equally active as bryostatin I in most in vitro studies (92, 93). This compound stands a better chance of being produced at larger scale by total synthesis.

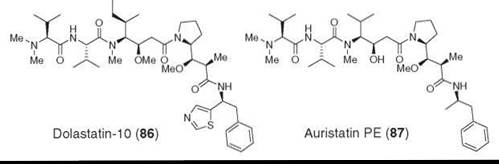
Dolastatins are a class of peptides comprised of mostly nonribosomal amino acids. They were isolated from a sea hare Dolabella auricularia (94). Dolastatin-10 (86) is one of the most potent and the best-studied members (95, 96). It exerts it antitumor effect by inhibiting tubulin polymerization and binds at the vinca alkaloid binding site (97, 98). Dolastatin-10 has been studied in Phase II human clinical trials but was discontinued because of lack of efficacy (99). Auristatin PE (87), a synthetic analog, seems to be more promising and is being studied in Phase II human trials (100).
Discodermolide (88) was isolated from Discodermia dissoluta by using a P388 cell line toxicity bioassay; later it was determined that it stabilized microtubule assembly better than Taxol®, and it drew a lot of attention as an anticancer agent (101, 102). Its development, like that of many other complex marine natural products, was hampered because of the lack of ample supply of the material required for the clinical studies. In this case, the supply problem was overcome by the synthetic efforts of the Novartis process group. They synthesized it on a large enough scale to allow clinical studies. Unfortunately, its development seems to have been halted because of toxicity at Phase I (103, 104).
Several other novel, structurally and mechanistically diverse marine natural products have entered various preclinical and clinical studies. One of these products is ecteinascidin-743 (89 ET-743), isolated from the tunicate Ecteinscidea turbinata (105, 106) and recently approved for the treatment of sarcoma in the European Union as the first “direct-from-the-sea” drug. Total synthesis and methods developed during the total synthesis allowed the preparation of a simpler analog phthalascidin (90) with comparable activities (107-109).
Hemiasterlin (91), a tripeptide isolated from a sponge that was chemically modified to HTI-286 (92), which binds to the vinca binding site of tubulin, depolarizes microtubules; it entered into clinical development but was apparently dropped (110, 111).
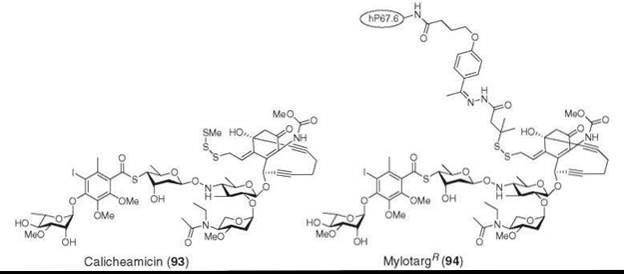
One difficulty with the cytotoxic agents that are used for the treatment of cancer is the differentiation of cytotoxicity between target tumor cells and normal cells. In an innovative approach, the Wyeth group took advantage of a tumor cell-specific drug delivery mechanism of antibodies. They conjugated calicheamicin (93), perhaps the best described member of the ene-diyne class of highly cytotoxic antitumor antibiotics produced by Actinomycetes, with recombinant humanized IgG4 kappa antibody and developed Mylotarg® Wyeth, Madison, NJ (94), which binds to CD33 antigens expressed on the surface of leukemia blasts. Mylotarg® is an effective and less toxic treatment of myeloid leukemia (112-115).

Immunosuppressant Agents
Natural products represent essentially all clinically used immunosuppressant agents. These agents collectively have made organ transplant possible. Cyclosporin (95) is an N-methyl cyclic peptide and originally was isolated from the fungus Trichoderma polysporum as an antifungal agent; almost immediately, the inhibition of T-cell proliferation and in vivo immunosuppressive properties were discovered and led to the development and approval of this molecule as a highly effective immunosuppressive agent (116-118). Natural products isolation of related compounds allowed the discovery of new congeners with reduced or no immunosuppressive activity in favor of antifungal and various other biological activities (e.g., antiparasitic activity) and asthma (119, 120). FK506 (96), a macrocyclic lactone, discovered from Streptomyces tsukubaensis as an immunosuppressive agent (121-125), was approved for clinical use for organ transplant as Tacrolimus® Astellas, Tokyo, Japan (126). Rapamycin (97), another macrocyclic lactone that is a very potent immunosuppressive agent, was approved as Sirolimus® Wyeth, Madison, NJ for clinical use for transplant rejection (127, 128). Rapamycin was isolated from Streptomyces hygroscopicus (129, 130). Mechanistically, all three of these compounds bind to their specific intracellular receptors, immunophilins, and the resulting complexes target the protein phosphatase, calcineurin (cyclosprin and FK506), and mammalian target of rapamycin (mTOR) to exert their immunosuppressive effects (131-133). Rapamycin and FK506 have played significant roles in studies of signal transduction and identification of various targets for other therapeutic applications, such as mTOR. Mycophenolic acid (98) originally was isolated from various species of Penicillium, and its antifungal activity has been known since 1932 (134). Mycophenolate was approved for acute rejection of kidney transplant (135, 136).
Cardiovascular Agents
The biggest impact made by natural products in the treatment of cardiovascular diseases is undoubtedly associated with the discovery of the first of the HMG CoA reductase inhibitors by enzyme-based screening of microbial extracts that led to the isolation (from Aspergillus terreus) and characterization of mevinolin (lovastatin 99), which is a homologue of compactin (100) that was discovered earlier (137, 138). These compounds possess a lipophilic hexahydrodecalin, a 2-methylbutanoate side chain, and a β-hydroxy-δ-lactone connected to the decalin unit with a two-carbon linker. These compounds are potent inhibitors of HMG CoA reductase, the rate-limiting enzyme of cholesterol biosynthesis, and inhibit the synthesis of cholesterol in the liver. Lovastatin (Mevacor®) Merck & Co., Inc., Whitehouse Station, NJ; was the first compound approved for lowering cholesterol in humans and became the cornerstone of all cholesterol-lowering agents generically called “statins.” The modification of 2-methylbutanoate to 2,2-dimethylbutanoate led to the semisynthetic derivative, simvastatin (Zocor®, Merck & Co., Inc., Whitehouse Station, NJ; 101), the second and more effective agent approved for human use (139). Hydroxylation of compactin by biotransformation led to pravastatin (Pravachol®, BMS, NY 102) (139). The key pharmacophore of the statins is the β-hydroxy-δ-lactone or open acid. As the importance and value of cholesterol lowering to human pathophysiology became clearer, the search for additional cholesterol-lowering agents became more prominent and led to the discovery and development of several other clinical agents. All these compounds retained nature’s gift of the pharmacophore, β-hydroxy-δ-lactone (or open acid), with replacement of the decalin unit of the natural products with a variety of aromatic lipophilic groups that resulted in fluvastin (103) (140), Atorvastatin (104) (141), Cerivastatin (105, withdrawn from the clinic) (142), Rosuvastatin (106) (143), and Pitavastatin (107). The statins have had tremendous impact in improvement of overall human health and quality of life because of the lowering of low-density lipoprotein (LDL) particles, which leads to a reduction in the incidence of coronary heart disease; arguably, they are the most successful class of medicines.
Ephedrine (108), isolated from the Chinese plant Ephedra sinaica, was approved as one of the first bronchodilators and cardiovascular agents. This discovery led to a variety of such antihypertensive agents including β-blockers (4).
Angiotensin-converting enzyme (ACE) converts angiotensin I to angiotensin II, and its inhibition has led to several very successful, clinically useful antihypertensive agents. Although these inhibitors are of synthetic origin, the original lead was modeled after a nonapeptide, teprotide (109). This peptide was isolated from snake (viper, Bothrops jararaca) venom by Ondetti et al. It had antihypertensive activity in the clinic by parenteral administration (138,144,145) but was devoid of oral activity. Ondetti and coworkers worked diligently, and, recognizing that ACE was a metallo-enzyme, they visualized the binding of a smaller snake-venom peptide SQ20475 (110) with ACE; they modeled an acyl-proline with a sulfhydryl substitution at the zinc binding site, which led to the design and synthesis of captopril (111) as an orally active highly effective antihypertensive clinical agent. Additional application of the rational design by Patchett and coworkers led to the synthesis of enalapril (112) and other clinically relevant oral ACE inhibitors (138).

Antiparasitic Agents
Avermectins (113, 114) are a series of macrocyclic lactones that are broad-spectrum, highly potent, glutamate-gated, chloride channel-modulator antiparasitic agents produced by Streptomyces avermitilis (146, 147). Ivermectin, 23,24-dihydroavermectin B1a/B1b (115), was the first product approved in the mid-1980s for treatment of intestinal parasites in domesticated and farm animals, and it remains the standard of care (148, 149). The remarkable activity of ivermectin against Onchocerca volvulus, the causative parasitic agent of onchocerciasis (river blindness), led to clinical development and the approval of Mectizan® Merck & Co., Inc., Whitehouse Station, NJ; for the treatment of such diseases. These parasitic diseases have debilitated millions of people in many countries in Africa and South America. Because Mectizan® is a very effective treatment, Merck is providing this drug free of cost to all people in need as a part of the “Mectizan Donation Program,” which has had tremendous impact on the health and quality of life of people affected by these diseases (150).
Spinosyns were discovered from the fermentation broth of Saccharopolyspora spinosa by screening for mortality of blowfly larvae, and a mixture of spinosyns A (116) and D (117) was approved and used successfully as a crop protection and an antiparasitic animal health agent. (151) Nodulisporic acids are an indole diterpenoid class discovered from various species of Nodulisporium as orally active antiflea and antitick agents for dogs and cats (152, 153). The most active of the series is nodulisporic acid A (118), which selectively modulates the activity of insect-specific glutamate-gated chloride channels (153).
Pharmaceutical Models
The roles played by natural products as models for design and development of pharmaceutical agents are too many to cover in this overview. A few examples are illustrated during the discussions of specific disease areas above. For example, a marine sponge-derived nucleoside was the precursor for various nucleoside-based antiviral agents, pepstatin for renin and HIV protease inhibitors, snake venom peptide for ACE inhibitors, lovastatin and compactin for all statins, and ephedrine for many painkillers and P-blockers. Below are a few critical examples that have played a big role in defining leads for some therapeutic areas but have not resulted in a drug yet.
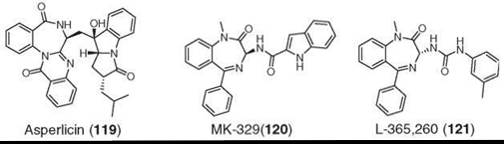
Asperlicin (119) was isolated from Aspergillus alliaceus as a weak cholecystokinin A receptor (CCK-A) antagonist by using CCK receptor binding screening assays (154). It is a competitive antagonist of CCK-A (but not CCK-B) but did not have sufficient potency or oral activity to qualify as a drug candidate. In a remarkable strategy, medicinal chemists simplified the molecule to a benzodiazepine core of asperlicin, which led to the synthesis of potent, safe, and orally active analogs (120 and 121) with selectivity for either CCK-A (120) or CCK-B (121) receptors. They entered human clinical trials but were abandoned because of lack of efficacy (155, 156). The benzodiazepine scaffold was coined as “privileged structures” by Evans et al (155). This discovery is a beautiful demonstration of how a natural product became a model for the CCK program and played a pivotal role in defining the entire field (138).
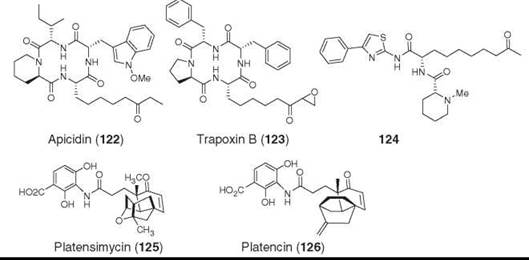
The second example is apicidin (122), which is a cyclic tetrapeptide isolated from a fungus Fusarium pallidoroseum by using an empiric antiprotozoal screen (157, 158). It showed potent inhibition of apicomplexan protozoa including the malarial parasite Plasmodium falciparum and coccidiosis parasite Eimeria spp. It was effective in vivo against reducing malaria parasite infection in a mouse model (157) and exhibited strong activity against tumor cell lines (159, 160). Cyclic tetrapeptides with a terminal epoxy-ketone were known to be effective cytotoxic agents before the discovery of apicidin, but the pharmacophore was associated with the epoxy-ketone group (e.g., Trapoxin B, 123) with covalent modification as a mode of action. Apicidin does not contain the epoxy-ketone but showed potent antitumor activity (161). The mode of action of apicidin was shown to be the inhibition of histone deacetylase (HDAC) (157). The amino-oxo-decanoic acid (L-Aoda) mimics the acetylated lysine residue and positions itself at the zinc-binding site of HDAC (162). Chemical modification of apicidin with retention of the ethyl or methyl ketone led to the synthesis of small dipeptides (e.g., 124) that retained the HDAC and tumor cell line inhibitory activities with significant reduction of inhibition of normal cells (161).
In summary, nature has provided a great set of molecules with enormous chemical diversity that has contributed to the treatment of many human diseases. Nature continues to amaze us with novel chemical diversity with unimaginable biological activity and target specificity, as illustrated by the recent discovery of the fatty acid synthesis inhibitor antibiotic platensimycin (125) (163, 164) and platencin (126) (165, 166). The former shows exquisite selectivity for FabF, whereas the latter compound is a balanced inhibitor of both condensing enzymes, FabF and FabH.
References
1. Butler M. The role of natural product chemistry in drug discovery. J. Nat. Prod. 2004; 67:2141-2153.
2. Clardy J, Walsh CT. Lessons from natural molecules. Nature 2004; 432:829-836.
3. Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981-2002. J. Nat. Prod. 2003; 66:1022-1037.
4. Newman DJ, Cragg GM, Snader KM. The influence of natural products upon drug discovery. Nat. Prod. Rep. 2000; 17:215-234.
5. Singh SB, Barrett JF. Empirical antibacterial drug discovery-foundation in natural products. Biochem. Pharmacol. 2006; 71:1006-1015.
6. Walsh CT. Antibiotics: Actions, Origin, Resistance. 2003. ASM Press, Washington, DC.
7. Albers-Schoenberg G, Arison BH, Hensens OD, Hirshfield J, Hoogsteen K, Kaczka EA, Rhodes RE, Kahan JS, Kahan FM, et al. Structure and absolute configuration of thienamycin. J. Am. Chem. Soc. 1978; 100:6491-6499.
8. Salzmann TN, Ratcliffe RW, Christensen BG, Bouffard FA. A stereocontrolled synthesis of +-thienamycin. J. Am. Chem. Soc. 1980; 102:6161-6163.
9. Zhanel GG, Wiebe R, Dilay L, Thomson K, Rubinstein E, Hoban DJ, Noreddin AM, Karlowsky JA. Comparative review of the carbapenems. Drugs 2007; 67:1027-1052.
10. White AR, Kaye C, Poupard J, Pypstra R, Woodnutt G, Wynne B. Augmentin amoxicillin/clavulanate in the treatment of community-acquired respiratory tract infection: a review of the continuing development of an innovative antimicrobial agent. J. Antimicrob. Chemother. 2004; 53:3-20.
11. Guay DR, Koskoletos C. Aztreonam, a new monobactam antimicrobial. Clin. Pharm. 1985; 4:516-526.
12. Rubinstein E, Vaughan D. Tigecycline: a novel glycylcycline. Drugs 2005; 65:1317-1336.
13. Yassin HM, Dever LL. Telithromycin: a new ketolide antimicrobial for treatment of respiratory tract infections. Expert. Opin. Investig. Drugs 2001; 10:353-367.
14. Woodford N. Novel agents for the treatment of resistant Grampositive infections. Expert. Opin. Investig. Drugs 2003; 12:117-137.
15. Bonfiglio G, Furneri PM. Novel streptogramin antibiotics. Expert. Opin. Investig. Drugs 2001; 10:185-198.
16. Georgopapadakou NH. Update on antifungals targeted to the cell wall: focus on beta-1,3-glucan synthase inhibitors. Expert. Opin. Investig. Drugs 2001; 10:269-280.
17. Denning DW. Echinocandin antifungal drugs. Lancet 2003; 362:1142-1151.
18. Vroman JA, Alvim-Gaston M, Avery MA. Current progress in the chemistry, medicinal chemistry and drug design of artemisinin based antimalarials. Curr. Pharm. Des. 1999; 5:101-138.
19. Ro DK, Paradise EM, Ouellet M, Fisher KJ, Newman KL, Ndungu JM, Ho KA, Eachus RA, Ham TS, Kirby J, Chang MC, Withers ST, Shiba Y, Sarpong R, Keasling JD. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 2006; 440:940-943.
20. Withers ST, Keasling JD. Biosynthesis and engineering of iso- prenoid small molecules. Appl. Microbiol. Biotechnol. 2007; 73:980-990.
21. Chang MC, Eachus RA, Trieu W, Ro DK, Keasling JD. Engineering Escherichia coli for production of functionalized terpenoids using plant P450s. Nat. Chem. Biol. 2007; 3:274-277.
22. Bergmann W, Feeney RJ. Contributions to the study of marine products. XXXII. The nucleosides of sponges I. J. Org. Chem. 1951; 16:981-987.
23. Bergmann W, Feeney RJ. The isolation of a new thymine pentoside from sponges. J. Am. Chem. Soc. 1950; 72:2809-2810.
24. Bergmann W, Burke DC. Contributions to the Study of Marine Products. XL. The nucleosides of sponges. IV. Spongosine. J. Org. Chem. 1956; 21:226-228.
25. Wiley RA, Rich DH. Peptidomimetics derived from natural products. Med. Res. Rev. 1993; 13:327-384.
26. Dorsey BD, McDonough C, McDaniel SL, Levin RB, Newton CL, Hoffman JM, Darke PL, Zugay-Murphy JA, Emini EA, Schleif WA, Olsen DB, Stahlhut MW, Rutkowski CA, Kuo LC, Lin JH, Chen IW, Michelson SR, Holloway MK, Huff JR, Vacca JP. Identification of MK-944a: a second clinical candidate from the hydroxylaminepentanamide isostere series of HIV protease inhibitors. J. Med. Chem. 2000; 43:3386-3399.
27. Terr CE, Pellens M. The Opium Problem. 1928. Bureau of Social Hygeine, New York:
28. Eddy NB, Friebel H, Hahn KJ, Halbach H. Codeine and its alternates for pain and cough relief. 2. Alternates for pain relief. Bull. World Health Organ. 1969; 40:1-53.
29. Heel RC, Brogden RN, Speight TM, Avery GS. Buprenorphine: a review of its pharmacological properties and therapeutic efficacy. Drugs 1979; 17:81-110.
30. Walker CS, Steel D, Jacobsen RB, Lirazan MB, Cruz LJ, Hooper D, Shetty R, DelaCruz RC, Nielsen JS, Zhou LM, Bandyopadhyay P, Craig AG, Olivera BM. The T-superfamily of conotoxins. J. Biol. Chem. 1999; 274:30664-30671.
31. Heading CE. Ziconotide Elan Pharmaceuticals. IDrugs 2001; 4:339-350.
32. Miljanich GP. Ziconotide: neuronal calcium channel blocker for treating severe chronic pain. Curr. Med. Chem. 2004; 11:3029- 3040.
33. Spande TF, Garraffo HM, Edwards MW, Yeh HJC, Pannell L, Daly JW. Epibatidine: a novel chloropyridylazabicycloheptane with potent analgesic activity from an Ecuadoran poison frog. J. Am. Chem. Soc. 1992; 114:3475-3478.
34. Daly JW. The chemistry of poisons in amphibian skin. Proc. Natl. Acad. Sci. U.S.A. 1995; 92:9-13.
35. Badio B, Daly JW. Epibatidine, a potent analgetic and nicotinic agonist. Mol. Pharmacol. 1994; 45:563-9.
36. Holladay MW, Bai H, Li Y, Lin NH, Daanen JF, Ryther KB, Wasicak JT, Kincaid JF, He Y, Hettinger AM, Huang P, Anderson DJ, Bannon AW, Buckley MJ, Campbell JE, Donnelly-Roberts DL, Gunther KL, Kim DJ, Kuntzweiler TA, Sullivan JP, Decker MW, Arneric SP. Structure-activity studies related to ABT-594, a potent nonopioid analgesic agent: effect of pyridine and azetidine ring substitutions on nicotinic acetylcholine receptor binding affinity and analgesic activity in mice. Bioorg. Med. Chem. Lett. 1998; 8:2797-2802.
37. Decker MW, Rueter LE, Bitner RS. Nicotinic acetylcholine receptor agonists: a potential new class of analgesics. Curr. Top. Med. Chem. 2004; 4:369-384.
38. Decker MW, Meyer MD, Sullivan JP. The therapeutic potential of nicotinic acetylcholine receptor agonists for pain control. Expert. Opin. Investig. Drugs 2001; 10:1819-1830.
39. Decker MW, Meyer MD. Therapeutic potential of neuronal nicotinic acetylcholine receptor agonists as novel analgesics. Biochem. Pharmacol. 1999; 58:917-923.
40. Hochuli E, Kupfer E, Maurer R, Meister W, Mercadal Y, Schmidt K. Lipstatin, an inhibitor of pancreatic lipase, produced by Streptomyces toxytricini. II. Chemistry and structure elucidation. J Antibiot. (Tokyo) 1987; 40:1086-1091.
41. Weibel EK, Hadvary P, Hochuli E, Kupfer E, Lengsfeld H. Lipstatin an inhibitor of pancreatic lipase, produced by Streptomyces toxytricini. I. Producing organism, fermentation, isolation and biological activity. J. Antibiot. (Tokyo) 1987; 40:1081-1085.
42. McNeely W, Benfield P. Orlistat. Drugs 1998; 56:241-9;250.
43. Mancino JM. Orlistat: Current issues for patients with type 2 diabetes. Curr. Diab. Rep. 2006; 6:389-394.
44. Henness S, Perry CM. Orlistat: a review of its use in the management of obesity. Drugs 2006; 66:1625-1656.
45. Miyazaki Y, Godaishi K. Experimental Cultivation of the Plants Containing Galanthamine at Izu. 1 General Growth of Shokiran Lycoris Aurea Herb., Natsuzuisen L. Squamigera Maxim., Snowflake Leucojum Aestivum L., and Snowdrop Galanthus Nivalis L., 1961 to 1962.. Eisei. Shikenjo. Hokoku. 1963; 81:172-176.
46. Thomsen T, Kewitz H. Selective inhibition of human acetylcholinesterase by galanthamine in vitro and in vivo. Life Sci. 1990; 46:1553-1558.
47. Greenblatt HM, Kryger G, Lewis T, Silman I, Sussman JL. Structure of acetylcholinesterase complexed with -galanthamine at 2.3 A resolution. FEBS Lett. 1999; 463:321-326.
48. Lee KH. Novel antitumor agents from higher plants. Med. Res. Rev. 1999; 19:569-596.
49. Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 1971; 93:2325-2327.
50. Schiff PB, Horwitz SB. Taxol assembles tubulin in the absence of exogenous guanosine 5’-triphosphate or microtubule-associated proteins. Biochemistry 1981; 20:3247-3252.
51. Schiff PB, Horwitz SB. Taxol stabilizes microtubules in mouse fibroblast cells. Proc. Natl. Acad. Sci. U.S.A. 1980; 77:1561-1565.
52. Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature 1979; 277:665-667.
53. Wall ME, Wani MC, Cook CE, Palmer KH, McPhail AT, Sim GA. Plant Antitumor Agents. I. The Isolation and Structure of Camptothecin, a Novel Alkaloidal Leukemia and Tumor Inhibitor from Camptotheca acuminata. J. Am. Chem. Soc. 1966; 88:3888-3890.
54. Srivastava V, Negi AS, Kumar JK, Gupta MM, Khanuja SP. Plant-based anticancer molecules: a chemical and biological profile of some important leads. Bioorg. Med. Chem. 2005; 13:5892- 5908.
55. Sandler A. Irinotecan therapy for small-cell lung cancer. Oncology 2002; 16:419-438.
56. Neuss N, Gorman M, Svoboda GH, Maciak G, Beer CT. Vinca alkaloids. III. Characterization of leurosine and vindaleukoblastine, new alkaloids from Vinca rosea Linn. J. Am. Chem. Soc. 1959; 81:4754-4755.
57. Moncrief JW, Lipscomb WN. Structures of leurocristine vincristine and vincaleukoblastine.1 X-Ray analysis of leurocristine methiodide. J. Am. Chem. Soc. 1965; 87:4963-4964.
58. Hochster HS, Vogel CL, Burman SL, White R. Activity and safety of vinorelbine combined with doxorubicin or fluorouracil as first-line therapy in advanced breast cancer: a stratified phase II study. Oncologist 2001; 6:269-277.
59. Barnett CJ, Cullinan GJ, Gerzon K, Hoying RC, Jones WE, Newlon WM, Poore GA, Robison RL, Sweeney MJ, et al. Structure-activity relationships of dimeric Catharanthus alkaloids. 1. Deacetyl vinblastine amide vindesine sulfate. J. Med. Chem. 1978; 21:88-96.
60. Sorensen JB, Hansen HH. Is there a role for vindesine in the treatment of non-small cell lung cancer? Invest. New Drugs 1993; 11:103-133.
61. Sorensen JB, Osterlind K, Hansen HH. Vinca alkaloids in the treatment of non-small cell lung cancer. Cancer Treat. Rev. 1987; 14:29-51.
62. Ruckdeschel JC. Etoposide in the management of non-small cell lung cancer. Cancer 1991; 67:2533.
63. Gordaliza M, Castro MA, del Corral JM, Feliciano AS. Antitumor properties of podophyllotoxin and related compounds. Curr. Pharm. Des. 2000; 6:1811-1839.
64. Baldwin EL, Osheroff N. Etoposide, topoisomerase II and cancer. Curr. Med. Chem. Anticancer Agents 2005; 5:363-372.
65. You Y. Podophyllotoxin derivatives: current synthetic approaches for new anticancer agents. Curr. Pharm. Des. 2005; 11:1695-1717.
66. Pettit GR, Singh SB, Hamel E, Lin CM, Alberts DS, Garcia-Kendall D. Isolation and structure of the strong cell growth and tubulin inhibitor combretastatin A-4. Experientia 1989; 45:209-211.
67. Lin CM, Singh SB, Chu PS, Dempcy RO, Schmidt JM, Pettit GR, Hamel E. Interactions of tubulin with potent natural and synthetic analogs of the antimitotic agent combretastatin: a structure-activity study. Mol. Pharmacol. 1988; 34:200-208.
68. Kirwan IG, Loadman PM, Swaine DJ, Anthoney DA, Pettit GR, Lippert JW, 3rd, Shnyder SD, Cooper PA, Bibby MC. Comparative preclinical pharmacokinetic and metabolic studies of the combretastatin prodrugs combretastatin A4 phosphate and A1 phosphate. Clin. Cancer Res. 2004; 10:1446-1453.
69. Nabha SM, Mohammad RM, Dandashi MH, Coupaye-Gerard B, Aboukameel A, Pettit GR, Al-Katib AM. Combretastatin-A4 prodrug induces mitotic catastrophe in chronic lymphocytic leukemia cell line independent of caspase activation and polyADP-ribose polymerase cleavage. Clin. Cancer Res. 2002; 8:2735-2741.
70. Chaplin DJ, Pettit GR, Hill SA. Anti-vascular approaches to solid tumour therapy: evaluation of combretastatin A4 phosphate. Anticancer Res. 1999; 19:189-195.
71. Dorr RT, Dvorakova K, Snead K, Alberts DS, Salmon SE, Pettit GR. Antitumor activity of combretastatin-A4 phosphate, a natural product tubulin inhibitor. Invest. New Drugs 1996; 14:131-137.
72. Phillips DR, White RJ, Cullinane C. DNA sequence-specific adducts of adriamycin and mitomycin C. FEBS Lett. 1989; 246:233-240.
73. Skorobogaty A, White RJ, Phillips DR, Reiss JA. Elucidation of the DNA sequence preferences of daunomycin. Drug. Des. Deliv. 1988; 3:125-151.
74. Skorobogaty A, White RJ, Phillips DR, Reiss JA. The 5’-CA DNA-sequence preference of daunomycin. FEBS Lett. 1988; 227:103-106.
75. Hecht SM. Bleomycin: new perspectives on the mechanism of action. J. Nat. Prod. 2000; 63:158-168.
76. White RJ, Durr FE. Development of mitoxantrone. Invest. New Drugs 1985; 3:85-93.
77. Sasaki Y, Seto M, Komatsu K, Omura S. Staurosporine, a protein kinase inhibitor, attenuates intracellular Ca2 + -dependent contractions of strips of rabbit aorta. Eur. J. Pharmacol. 1991; 202:367-372.
78. Omura S. The expanded horizon for microbial metabolites-a review. Gene 1992; 115:141-149.
79. Omura S, Sasaki Y, Iwai Y, Takeshima H. Staurosporine, a potentially important gift from a microorganism. J. Antibiot. (Tokyo) 1995; 48:535-548.
80. Thavasu P, Propper D, McDonald A, Dobbs N, Ganesan T, Talbot D, Braybrook J, Caponigro F, Hutchison C, Twelves C, Man A, Fabbro D, Harris A, Balkwill F. The protein kinase C inhibitor CGP41251 suppresses cytokine release and extracellular signal-regulated kinase 2 expression in cancer patients. Cancer Res. 1999; 59:3980-3984.
81. Propper DJ, McDonald AC, Man A, Thavasu P, Balkwill F, Braybrooke JP, Caponigro F, Graf P, Dutreix C, Blackie R, Kaye SB, Ganesan TS, Talbot DC, Harris AL, Twelves C. Phase I and pharmacokinetic study of PKC412, an inhibitor of protein kinase C. J. Clin. Oncol. 2001; 19:1485-1492.
82. Gerth K, Bedorf N, Hofle G, Irschik H, Reichenbach H. Epothilons A and B: antifungal and cytotoxic compounds from Sorangium cellulosum Myxobacteria. Production, physicochemical and biological properties. J. Antibiot. (Tokyo) 1996; 49:560-563.
83. Bollag DM. Epothilones: novel microtubule-stabilising agents. Expert. Opin. Investig. Drugs 1997; 6:867-873.
84. Bollag DM, McQueney PA, Zhu J, Hensens O, Koupal L, Liesch J, Goetz M, Lazarides E, Woods CM. Epothilones, a new class of microtubule-stabilizing agents with a taxol-like mechanism of action. Cancer Res. 1995; 55:2325-2333.
85. Altmann KH, Pfeiffer B, Arseniyadis S, Pratt BA, Nicolaou KC. The Chemistry and Biology of Epothilones-The Wheel Keeps Turning. ChemMedChem 2007; 2:396-423.
86. Feling RH, Buchanan GO, Mincer TJ, Kauffman CA, Jensen PR, Fenical W. Salinosporamide A: a highly cytotoxic proteasome inhibitor from a novel microbial source, a marine bacterium of the new genus salinispora. Angew. Chem. Int. Ed. Engl. 2003; 42:355-357.
87. Groll M, Huber R, Potts BC. Crystal structures of Salinosporamide A NPI-0052 and B NPI-0047 in complex with the 20S proteasome reveal important consequences of beta-lactone ring opening and a mechanism for irreversible binding. J. Am. Chem. Soc. 2006; 128:5136-5141.
88. Macherla VR, Mitchell SS, Manam RR, Reed KA, Chao TH, Nicholson B, Deyanat-Yazdi G, Mai B, Jensen PR, Fenical WF, Neuteboom ST, Lam KS, Palladino MA, Potts BC. Structure-activity relationship studies of salinosporamide A NPI- 0052, a novel marine derived proteasome inhibitor. J. Med. Chem. 2005; 48:3684-3687.
89. Chauhan D, Catley L, Li G, Podar K, Hideshima T, Velankar M, Mitsiades C, Mitsiades N, Yasui H, Letai A, Ovaa H, Berkers C, Nicholson B, Chao TH, Neuteboom ST, Richardson P, Palladino MA, Anderson KC. A novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from Bortezomib. Cancer Cell 2005; 8:407-419.
90. Pettit GR, Herald CL, Doubek DL, Herald DL, Arnold E, Clardy J. Isolation and structure of bryostatin 1. J. Am. Chem. Soc. 1982; 104:6846-6848.
91. Hennings H, Blumberg PM, Pettit GR, Herald CL, Shores R, Yuspa SH. Bryostatin 1, an activator of protein kinase C, inhibits tumor promotion by phorbol esters in SENCAR mouse skin. Carcinogenesis 1987; 8:1343-1346.
92. Baryza JL, Brenner SE, Craske ML, Meyer T, Wender PA. Simplified analogs of bryostatin with anticancer activity display greater potency for translocation of PKCdelta-GFP. Chem. Biol. 2004; 11:1261-1267.
93. Wender PA, Hinkle KW, Koehler MF, Lippa B. The rational design of potential chemotherapeutic agents: synthesis of bryostatin analogues. Med. Res. Rev. 1999; 19:388-407.
94. Pettit GR. Th dolastatins. Fortschr Chem. Org. Naturst. 1997; 70:1-79.
95. Pettit GR, Kamano Y, Herald CL, Tuinman AA, Boettner FE, Kizu H, Schmidt JM, Baczynskyj L, Tomer KB, Bontems RJ. The isolation and structure of a remarkable marine animal antineoplastic constituent: dolastatin 10. J. Am. Chem. Soc. 1987; 109:6883-6885.
96. Pettit GR, Singh SB, Hogan F, Lloyd-Williams P, Herald DL, Burkett DD, Clewlow PJ. Antineoplastic agents. Part 189. The absolute configuration and synthesis of natural-dolastatin 10. J. Am. Chem. Soc. 1989; 111:5463-5465.
97. Bai R, Pettit GR, Hamel E. Dolastatin 10, a powerful cytostatic peptide derived from a marine animal. Inhibition of tubulin polymerization mediated through the vinca alkaloid binding domain. Biochem. Pharmacol. 1990; 39:1941-1949.
98. Bai RL, Pettit GR, Hamel E. Binding of dolastatin 10 to tubulin at a distinct site for peptide antimitotic agents near the exchangeable nucleotide and vinca alkaloid sites. J. Biol. Chem. 1990; 265:17141-17149.
99. Saad ED, Kraut EH, Hoff PM, Moore DF, Jr., Jones D, Pazdur R, Abbruzzese JL. Phase II study of dolastatin-10 as first-line treatment for advanced colorectal cancer. Am. J. Clin. Oncol. 2002; 25:451-453.
100. Patel S, Keohan ML, Saif MW, Rushing D, Baez L, Feit K, DeJager R, Anderson S. Phase II study of intravenous TZT-1027 in patients with advanced or metastatic soft-tissue sarcomas with prior exposure to anthracycline-based chemotherapy. Cancer 2006; 107:2881-2887.
101. Gunasekera SP, Gunasekera M, Longley RE, Schulte GK. Discodermolide: a new bioactive polyhydroxylated lactone from the marine sponge Discodermia dissoluta. J. Org. Chem. 1990; 55:4912-4915.
102. ter Haar E, Kowalski RJ, Hamel E, Lin CM, Longley RE, Gunasekera SP, Rosenkranz HS, Day BW. Discodermolide, a cytotoxic marine agent that stabilizes microtubules more potently than taxol. Biochemistry 1996;35:243-250.
103. Mickel SJ. Toward a commercial synthesis of +-discodermolide. Curr. Opin. Drug Discov. Devel. 2004; 7:869-881.
104. Mickel SJ, Niederer D, Daeffler R, Osmani A, Kuesters E, Schmid E, Schaer K, Gamboni R, Chen W, Loeser E, Kinder FR, Konigsberger K, Prasad K, Ramsey TM, Repic O, Wang R-M, Florence G, Lyothier I, Paterson I. Large-scale synthesis of the anti-cancer marine natural product +-discodermolide. Part 5: linkage of fragments C and Finale. Org. Process Res. Dev. 2004; 8:122-130.
105. Wright AE, Forleo DA, Gunawardana GP, Gunasekera SP, Koehn FE, McConnell OJ. Antitumor tetrahydroisoquinoline alkaloids from the colonial ascidian Ecteinascidia turbinata. J. Org. Chem. 1990; 55:4508-4512.
106. Rinehart KL, Holt TG, Fregeau NL, Stroh JG, Keifer PA, Sun F, Li LH, Martin DG. Ecteinascidins 729, 743, 745, 759A, 759B, and 770: potent antitumor agents from the Caribbean tunicate Ecteinascidia turbinata. J. Org. Chem. 1990; 55:4512-4515.
107. Corey EJ, Gin DY, Kania RS. Enantioselective total synthesis of ecteinascidin 743. J. Am. Chem. Soc. 1996; 118:9202-9203.
108. Martinez EJ, Corey EJ. A new, more efficient, and effective process for the synthesis of a key pentacyclic intermediate for production of ecteinascidin and phthalascidin antitumor agents. Org. Lett. 2000; 2:993-996.
109. Martinez EJ, Owa T, Schreiber SL, Corey EJ. Phthalascidin, a synthetic antitumor agent with potency and mode of action comparable to ecteinascidin 743. Proc. Natl. Acad. Sci. U.S.A. 1999; 96:3496-3501.
110. Anderson HJ, Coleman JE, Andersen RJ, Roberge M. Cytotoxic peptides hemiasterlin, hemiasterlin A and hemiasterlin B induce mitotic arrest and abnormal spindle formation. Cancer Chemother. Pharmacol. 1997; 39:223-226.
111. Loganzo F, Discafani CM, Annable T, Beyer C, Musto S, Hari M, Tan X, Hardy C, Hernandez R, Baxter M, Singanallore T, Khafizova G, Poruchynsky MS, Fojo T, Nieman JA, Ayral-Kaloustian S, Zask A, Andersen RJ, Greenberger LM. HTI-286, a synthetic analogue of the tripeptide hemiasterlin, Is a potent antimicrotubule agent that circumvents P-Glycoprotein-mediated resistance in vitro and in Vivo. Cancer Res. 2003; 63:1838-1845.
112. Maiese WM, Lechevalier MP, Lechevalier HA, Korshalla J, Kuck N, Fantini A, Wildey MJ, Thomas J, Greenstein M. Calicheamicins, a novel family of antitumor antibiotics: taxonomy, fermentation and biological properties. J. Antibiot. (Tokyo) 1989; 42:558-563.
113. Giles F, Estey E, O’Brien S. Gemtuzumab ozogamicin in the treatment of acute myeloid leukemia. Cancer 2003; 98:2095-2104.
114. Lee MD, Dunne TS, Siegel MM, Chang CC, Morton GO, Borders DB. Calichemicins, a novel family of antitumor antibiotics. 1. Chemistry and partial structure of calichemicin.gamma.1I. J. Am. Chem. Soc. 1987; 109:3464-3466.
115. Lee MD, Dunne TS, Chang CC, Ellestad GA, Siegel MM, Morton GO, McGahren WJ, Borders DB. Calichemicins, a novel family of antitumor antibiotics. 2. Chemistry and structure of cali- chemicin.gamma.1I. J. Am. Chem. Soc. 1987; 109:3466-3468.
116. Ruegger A, Kuhn M, Lichti H, Loosli HR, Huguenin R, Quiquerez C, von Wartburg A. Cyclosporin A, a peptide metabolite from trichoderma polysporum link ex pers. Rifai, with a remarkable immunosuppressive activity. Helv. Chim. Acta 1976; 59:1075-1092.
117. Wenger RM. Pharmacology of cyclosporin sandimmune. II. Chemistry. Pharmacol. Rev. 1990; 41:243-247.
118. Zenke G, Baumann G, Wenger R, Hiestand P, Quesniaux V, Andersen E, Schreier MH. Molecular mechanisms of immunosuppression by cyclosporins. Ann. N. Y. Acad. Sci. 1993; 685:330- 335.
119. Bua J, Ruiz AM, Potenza M, Fichera LE. In vitro anti-parasitic activity of Cyclosporin A analogs on Trypanosoma cruzi. Bioorg. Med. Chem. Lett. 2004; 14:4633-4637.
120. Eckstein JW, Fung J. A new class of cyclosporin analogues for the treatment of asthma. Expert. Opin. Investig. Drugs 2003; 12:647-653.
121. Kino T, Hatanaka H, Miyata S, Inamura N, Nishiyama M, Yajima T, Goto T, Okuhara M, Kohsaka M, Aoki H, et al. FK-506, a novel immunosuppressant isolated from a Streptomyces. II. Immunosuppressive effect of FK-506 in vitro. J. Antibiot. (Tokyo) 1987; 40:1256-1265.
122. Kino T, Inamura N, Sakai F, Nakahara K, Goto T, Okuhara M, Kohsaka M, Aoki H, Ochiai T. Effect of FK-506 on human mixed lymphocyte reaction in vitro. Transplant. Proc. 1987; 19:36-39.
123. Ochiai T, Nagata M, Nakajima K, Suzuki T, Sakamoto K, Enomoto K, Gunji Y, Uematsu T, Goto T, Hori S, et al. Studies of the effects of FK506 on renal allografting in the beagle dog. Transplantation 1987; 44:729-733.
124. Kino T, Goto T. Discovery of FK-506 and update. Ann. N. Y. Acad. Sci. 1993; 685:13-21.
125. Tanaka H, Kuroda A, Marusawa H, Hatanaka H, Kino T, Goto T, Hashimoto M, Taga T. Structure of FK506, a novel immunosuppressant isolated from Streptomyces. J. Am. Chem. Soc. 1987; 109:5031-5033.
126. Spencer CM, Goa KL, Gillis JC. Tacrolimus. An update of its pharmacology and clinical efficacy in the management of organ transplantation. Drugs 1997; 54:925-975.
127. Kahan BD, Camardo JS. Rapamycin: clinical results and future opportunities. Transplantation 2001; 72:1181-1193.
128. Camardo J. The Rapamune era of immunosuppression 2003: the journey from the laboratory to clinical transplantation. Transplant. Proc. 2003; 35:18-24.
129. Baker H, Sidorowicz A, Sehgal SN, Vezina C. Rapamycin AY-22,989, a new antifungal antibiotic. III. In vitro and in vivo evaluation. J. Antibiot. (Tokyo) 1978; 31:539-545.
130. Sehgal SN, Baker H, Vezina C. Rapamycin AY-22,989, a new antifungal antibiotic. II. Fermentation, isolation and characterization. J. Antibiot. (Tokyo) 1975; 28:727-732.
131. Schreiber SL. Chemistry and biology of the immunophilins and their immunosuppressive ligands. Science 1991; 251:283-287.
132. Ho S, Clipstone N, Timmermann L, Northrop J, Graef I, Fiorentino D, Nourse J, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Clin. Immunol. Immunopathol. 1996; 80:40-45.
133. Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol. Today 1992; 13:136-142.
134. Campbell IM, Calzadilla CH, McCorkindale NJ. Some new metabolites related to mycophenolic acid. Tetrahedron Lett. 1966: 5107-5111.
135. Behrend M. Mycophenolate mofetil Cellcept. Expert Opin. Investig. Drugs 1998; 7:1509-1519.
136. Sollinger HW. Mycophenolate mofetil for the prevention of acute rejection in primary cadaveric renal allograft recipients. U.S. Renal Transplant Mycophenolate Mofetil Study Group. Transplantation 1995; 60:225-232.
137. Alberts AW, Chen J, Kuron G, Hunt V, Huff J, Hoffman C, Rothrock J, Lopez M, Joshua H, Harris E, Patchett A, Monaghan R, Currie S, Stapley E, Albers-Schonberg G, Hensens O, Hirsh- field J, Hoogsteen K, Liesch J, Springer J. Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc. Natl. Acad. Sci. U.S.A. 1980; 77:3957-3961.
138. Patchett AA. 2002 Alfred Burger Award Address in Medicinal Chemistry. Natural products and design: interrelated approaches in drug discovery. J. Med. Chem. 2002; 45:5609-5616.
139. Coukell AJ, Wilde MI. Pravastatin. A pharmacoeconomic review of its use in primary and secondary prevention of coronary heart disease. Pharmacoeconomics 1998; 14:217-236.
140. Plosker GL, Wagstaff AJ. Fluvastatin: a review of its pharmacology and use in the management of hypercholesterolaemia. Drugs 1996; 51:433-459.
141. Roth BD. The discovery and development of atorvastatin, a potent novel hypolipidemic agent. Prog. Med. Chem. 2002; 40:1-22.
142. Plosker GL, Dunn CI, Figgitt DP. Cerivastatin: a review of its pharmacological properties and therapeutic efficacy in the management of hypercholesterolaemia. Drugs 2000; 60:1179-1206.
143. Chong PH, Yim BT. Rosuvastatin for the treatment of patients with hypercholesterolemia. Ann. Pharmacother. 2002; 36:93-101.
144. Ondetti MA, Williams NJ, Sabo EF, Pluscec J, Weaver ER, Kocy O. Angiotensin-converting enzyme inhibitors from the venom of Bothrops jararaca. Isolation, elucidation of structure, and synthesis. Biochemistry 1971; 10:4033-4039.
145. Ondetti MA, Rubin B, Cushman DW. Design of specific inhibitors of angiotensin-converting enzyme: new class of orally active antihypertensive agents. Science 1977; 196:441-444.
146. Burg RW, Miller BM, Baker EE, Birnbaum J, Currie SA, Hartman R, Kong YL, Monaghan RL, Olson G, Putter I, Tunac JB, Wallick H, Stapley EO, Oiwa R, Omura S. Avermectins, new family of potent anthelmintic agents: producing organism and fermentation. Antimicrob, Agents Chemother. 1979; 15:361-367.
147. Cully DF, Vassilatis DK, Liu KK, Paress PS, Van der Ploeg LH, Schaeffer JM, Arena JP. Cloning of an avermectin-sensitive glutamate-gated chloride channel from Caenorhabditis elegans. Nature 1994; 371:707-711.
148. Davies HG, Green RH. Avermectins and milbemycins. Nat. Prod. Rep. 1986; 3:87-121.
149. Chabala JC, Mrozik H, Tolman RL, Eskola P, Lusi A, Peterson LH, Woods MF, Fisher MH, Campbell WC, Egerton JR, Ostlind DA. Ivermectin, a new broad-spectrum antiparasitic agent. J. Med. Chem. 1980; 23:1134-1136.
150. Goa KL, McTavish D, Clissold SP. Ivermectin. A review of its antifilarial activity, pharmacokinetic properties and clinical efficacy in onchocerciasis. Drugs 1991; 42:640-658.
151. Kirst HA, Creemer LC, Naylor SA, Pugh PT, Snyder DE, Winkle JR, Lowe LB, Rothwell JT, Sparks TC, Worden TV. Evaluation and development of spinosyns to control ectoparasites on cattle and sheep. Curr. Top. Med. Chem. 2002; 2:675-699.
152. Ondeyka JG, Helms GL, Hensens OD, Goetz MA, Zink DL, Tsipouras A, Shoop WL, Slayton L, Dombrowski AW, Polishook JD, Ostlind DA, Tsou NN, Ball RG, Singh SB. Nodulisporic Acid A, a novel and potent insecticide from a nodulisporium sp. Isolation, structure determination, and chemical transformations. J. Am. Chem. Soc. 1997; 119:8809-8816.
153. Meinke PT, Smith MM, Shoop WL. Nodulisporic acid: its chemistry and biology. Curr. Top. Med. Chem. 2002; 2:655-674.
154. Chang RS, Lotti VJ, Monaghan RL, Birnbaum J, Stapley EO, Goetz MA, Albers-Schonberg G, Patchett AA, Liesch JM, Hensens OD, et al. A potent nonpeptide cholecystokinin antagonist selective for peripheral tissues isolated from Aspergillus alliaceus. Science 1985; 230:177-179.
155. Evans BE, Bock MG, Rittle KE, DiPardo RM, Whitter WL, Veber DF, Anderson PS, Freidinger RM. Design of potent, orally effective, nonpeptidal antagonists of the peptide hormone chole- cystokinin. Proc. Natl. Acad. Sci. U.S.A. 1986; 83:4918-4922.
156. Bock MG, DiPardo RM, Evans BE, Rittle KE, Whitter WL, Veber DE, Anderson PS, Freidinger RM. Benzodiazepine gastrin and brain cholecystokinin receptor ligands: L-365,260. J. Med. Chem. 1989; 32:13-16.
157. Darkin-Rattray SJ, Gurnett AM, Myers RW, Dulski PM, Crumley TM, Allocco JJ, Cannova C, Meinke PT, Colletti SL, Bednarek MA, Singh SB, Goetz MA, Dombrowski AW, Polishook JD, Schmatz DM. Apicidin: a novel antiprotozoal agent that inhibits parasite histone deacetylase. Proc. Natl. Acad. Sci. U.S.A. 1996; 93:13143-13147.
158. Singh SB, Zink DL, Liesch JM, Mosley RT, Dombrowski AW, Bills GF, Darkin-Rattray SJ, Schmatz DM, Goetz MA. Structure and chemistry of apicidins, a class of novel cyclic tetrapeptides without a terminal alpha-keto epoxide as inhibitors of histone deacetylase with potent antiprotozoal activities. J. Org. Chem. 2002; 67:815-825.
159. Han JW, Ahn SH, Park SH, Wang SY, Bae GU, Seo DW, Kwon HK, Hong S, Lee HY, Lee YW, Lee HW. Apicidin, a histone deacetylase inhibitor, inhibits proliferation of tumor cells via induction of p21WAF1/Cip1 and gelsolin. Cancer Res. 2000; 60:6068-6074.
160. Kim MS, Son MW, Kim WB, In Park Y, Moon A. Apicidin, an inhibitor of histone deacetylase, prevents H-ras-induced invasive phenotype. Cancer Lett. 2000; 157:23-30.
161. Jones P, Altamura S, Chakravarty PK, Cecchetti O, De Francesco R, Gallinari P, Ingenito R, Meinke PT, Petrocchi A, Rowley M, Scarpelli R, Serafini S, Steinkuhler C. A series of novel, potent, and selective histone deacetylase inhibitors. Bioorg. Med. Chem. Lett. 2006; 16:5948-5952.
162. Remiszewski SW. Recent advances in the discovery of small molecule histone deacetylase inhibitors. Curr. Opin. Drug Discov. Devel. 2002; 5:487-499.
163. Singh SB, Jayasuriya H, Ondeyka JG, Herath KB, Zhang C, Zink DL, Tsou NN, Ball RG, Basilio A, Genilloud O, Diez MT, Vicente F, Pelaez F, Young K, Wang J. Isolation, structure, and absolute stereochemistry of platensimycin, a broad spectrum antibiotic discovered using an antisense differential sensitivity strategy. J. Am. Chem. Soc. 2006; 128:11916-11920.
164. Wang J, Soisson SM, Young K, Shoop W, Kodali S, Galgoci A, Painter R, Parthasarathy G, Tang YS, Cummings R, Ha S, Dorso K, Motyl M, Jayasuriya H, Ondeyka J, Herath K, Zhang C, Hernandez L, Allocco J, Basilio A, Tormo JR, Genilloud O, Vicente F, Pelaez F, Colwell L, Lee SH, Michael B, Felcetto T, Gill C, Silver LL, Hermes JD, Bartizal K, Barrett J, Schmatz D, Becker JW, Cully D, Singh SB. Platensimycin is a selective FabF inhibitor with potent antibiotic properties. Nature 2006; 441:358-361.
165. Wang J, Kodali S, Lee SH, Galgoci A, Painter R, Dorso K, Racine F, Motyl M, Hernandez L, Tinney E, Colletti SL, Herath K, Cummings R, Salazar O, Gonzalez I, Basilio A, Vicente F, Genilloud O, Pelaez F, Jayasuriya H, Young K, Cully DF, Singh SB. Discovery of platencin, a dual FabF and FabH inhibitor with in vivo antibiotic properties. Proc. Natl. Acad. Sci. U.S.A 2007; 104:7612-7616.
166. Jayasuriya H, Herath KB, Zhang C, Zink DL, Basilio A, Ge- nilloud O, Diez MT, Vicente F, Gonzalez I, Salazar O, Pelaez F, Cummings R, Ha S, Wang J, Singh SB. Isolation and structure of platencin: a FabH and FabF dual inhibitor with potent broad-spectrum antibiotic activity. Angew. Chem. Int. Ed. Engl. 2007; 46:4684-4688.