CHEMICAL BIOLOGY
Lipids, Organization and Aggregation of
Ilpo Vattulainen, Institute of Physics, Tampere University of Technology, Finland; Laboratory of Physics and Helsinki Institute of Physics, Helsinki University of Technology, Finland; MEMPHYS—Center for Biomembrane Physics, University of Southern Denmark, Odense, Denmark
doi: 10.1002/9780470048672.wecb664
Lipids constitute one of the main classes of molecules in biological systems. They are involved in numerous cellular functions either as individual molecules or as lipid aggregates with varying sizes and morphologies. For example, lipids are a crucial component of cellular membranes that surround and protect cells. Lipids comprise membrane domains that provide membrane proteins with a well-defined environment to carry out their functions. Lipids also interact specifically with some proteins, which render their functions possible. Lipids play the role of drugs and enzymes, and our skin and lung surfactant lining lung epithelial cells are composed largely of lipids. What is more, lipids are used in delivery vehicles to encompass drugs and other molecules. These functions and many others develop in part from lipids' specific properties relevant on molecular scales and also from the assembly of lipids as fascinating structures observed over a multitude of scales beyond molecular size. Here, we discuss the functions of lipids and lipid structures together with their structural and dynamic properties, including examples and highlights of recent studies.
Lipids (see Fig. 1) (1) constitute one of five classes of molecules that can be considered as crucial in biological systems. Together with proteins, nucleotides (DNA), carbohydrates, and water, lipids can be thought of as one of the basic building blocks of living systems.
For some reason, lipids cause a lot of emotions among the people. One often talks about “bad” and “good” fats, and in terms of health, this view is partly understandable. In a similar manner, it is common to talk about “bad” and “good” cholesterol; although in this case, most common people probably do not even know that only one type of cholesterol molecule exists, which is crucial for life. It is one of the most common and the most important lipids in eukaryotic cells. The “bad” and “good” cholesterols refer to the carrier particles (lipoproteins) that transport cholesterol molecules, and their effects on our health depend on the lipoprotein in question (2). Meanwhile, when a great deal of recent discussion has dealt with the genetic code and the proteomics of proteins, it seems that far less attention has been paid to the importance of lipids. In part, this attention is because no genes code lipids. Yet undoubtedly lipids are a crucial component of cells: They would not survive without lipids. It has been observed that polyunsaturated lipids that have several double bonds in their hydrocarbon chains are involved in the functioning of the eye, and DHA (perhaps the most important polyunsaturated fatty acid) is vital for normal brain development for infants and for the maintenance of normal brain function throughout life (3). Furthermore, we all appreciate the importance of lipids as a major source of energy, which may even be critical for survival under extreme conditions. In summary, lipids and fat are good. Those who love good food, such as sushi and fish in general, probably appreciate this view.
Appreciating the importance of lipids in a variety of biologically important cases, one is tempted to understand how they actually function and how that is affected by the structures of their assemblies. The answers to these questions are far from being resolved, but we do have some insight into the related issues. What is remarkable and deserves to be stressed here is that lipids are characterized by fascinating structures over a multitude of scales, which range from the size of individual molecules to the sizes of colloidal systems that comprise large amounts of lipid molecules (3): Lipids are the main component of lipoproteins that carry cholesterol, vesicles that transport molecules inside them, cell membranes that surround cells, lung surfactant that keeps us alive by allowing us to consume oxygen, and skin that protects us from the outside (4). Beautiful examples of the various lipid structures that range from micelles to vesicles and considerably more complicated morphologies are illustrated in References 3-7. It is truly fascinating to realize the remarkable diversity of lipid structures and to use that as a basis for the understanding of lipid functionality in biological systems.
Our aim in this article is to provide the reader with some flavor of the various structures and functions related to lipids. Rather than presenting an exhaustive description of the topic, however, we prefer a down-to-earth approach on a level in which we combine many relevant views and methods with several instructive examples and highlights of recent studies. The concise list of references at the end of this article follows this idea, in accord with the style of this review series. The emphasis will be on lipid membranes because of their abundance in numerous biological systems, which include cellular membranes, liposomes, and other delivery vehicles.

Figure 1. Examples of lipids found commonly and used in cells. (a) DPPC;(b) 1-palmitoyl-2-(1-pyrenedecanoyl)-sn-glycero-3-phosphocholine (PyrPC); (c) Palmitoyl-SM; (d) Cholesterol; (e) Dipalmitoylphosphatidylethanolamine; (f) 1,2-di-O-palmitoyl-3-O-β-D-galactosyl-sn-glycerol (DPGALA). Of these lipids, PyrPC is a pyrene-linked lipid probe and DPGALA is a glycolipid. The first four lipids are represented by a united-atom description, and the last two lipids are represented by a full-atom description.
Biological Relevance and Scales of Lipid Systems
The biological applications in which lipids are involved are numerous and profoundly important. Let us here consider some relevant applications.
Functions of lipids
No strict definition exists for the term “lipid” that is generally accepted. In a broad sense, lipids are compounds of low or intermediate molecular weight with a substantial proportion of hydrocarbons (1). Lipids have also been defined as “fatty acids and their derivatives, and substances related biosynthetically or functionally to these compounds” (4). Sometimes, it is also expected that molecules considered as lipids have some biological function. Many molecules based on fatty acids are lipids, but some vitamins and hormones are based on fatty acids as well. They serve many functions in living organisms, and the broad scope of these functions is truly fascinating.
The lipid membrane provides shelter for membrane proteins to do their functions. However, instead of working alone, membrane proteins such as ion channels work together with the membrane, such that the lipid composition around the protein actually affects the activation and the functioning of the protein. This idea is largely the essence of the lipid raft model (8), which highlights the importance of lipids in a variety of cellular functions. It has been observed, for example, that rhodopsin, which is the light sensitive membrane protein, favors interactions with polyunsaturated lipids (9).
Lipids are the main component in lipoproteins known as carriers of cholesterol (2). HDL (high-density lipoprotein, which is the “good” cholesterol) transports cholesterol and its esters from cells to liver for recirculation, whereas LDL (low-density lipoprotein, which is the “bad” cholesterol) carries cholesterol and cholesterol esters to the cells. The ratio of the two carrier particles partly determines one’s risk for diseases such as atherosclerosis, although the understanding and the overall view of the related issues is still incomplete in many ways.
Some lipid species serve as second messengers that pass on signals and information in the cell, such as in programmed cell death in which a lipid known as ceramide has been proposed to be the messenger (10). The same lipid is often found in hair conditioners. Lipids also play the role of enzymes, receptors, and drugs, and our skin is largely composed of lipids. In a similar manner, a major amount of molecules that comprise the pulmonary surfactant, which is a thin liquid film that lines lung epithelial cells, are saturated lipids (11) characterized by a lack of double bonds in the chains. The lung surfactant stabilizes the alveoli during expiration, when lungs undergo compression, and reduces the re-expansion work during inhalation. Lipids are also used widely for storing energy in terms of triglycerides, and recently, disorder in the lipid spectrum of cells has been related to, for example, atherosclerosis and major psychiatric diseases. As for applications, lipids are used by nature as novel micro-encapsulation devices—which is an exciting application nowadays used for drug and gene delivery (12).
Scales of lipid systems
Temperature scales
The relevant temperature range for functions that deal with lipids is the physiologic temperature, which is about 310 K. The phase behavior of lipid systems should then be considered with respect to this value. Particular attention is usually paid to the main transition temperature TM defined for a one-component lipid bilayer (see Fig. 2). Above TM, one finds a fluid (liquid-disordered) phase characterized by a lack of translational order in the bilayer plane and weak ordering of the lipid hydrocarbon chains. Below TM in the gel phase, in turn, the lipids in the membrane plane position themselves to follow hexagonal packing, besides which the hydrocarbon chains are strongly ordered (all-trans) and tilted with respect to the membrane normal direction. These transitions in lipid conformational and translational order can be characterized readily by order parameters such as the second-order Legendre polynomial and the in-plane structure factor (13, 14). The second-order Legendre polynomial is used often because it can be exploited to provide insight into the orientation of lipid hydrocarbon chains, their tilt as well as the orientation of a lipid head group, and it can be accessed rather easily through simulations as well as experiments [nuclear magnetic resonance (NMR), see below]. The main transition temperature varies from one lipid to another and is largest for lipids with saturated and long chains. For example for a dipalmitoylsphosphatidylcholine (DPPC) bilayer, it is 314.5 K. For comparison, for polyunsaturated lipids typical main transition temperatures are below 273 K. In a similar manner, other lipid systems such as droplets of triglycerides have distinctly different phases, usually crystalline at low temperatures and fluid above the transition point. For most of the physiologically important lipids TM is below 310 K, which implies that the physiologically more important phase is the fluid phase.
However, natural membranes are not one-component bilayers but rather are comprised of a variety of different lipid components. This matter is discussed below in more detail. Consequently, the presence of many lipid types develops a formation of membrane domains with varying composition, and hence the main phase transition temperature is not well defined for a many-component membrane. Of particular interest are membranes with a large concentration of cholesterol, because cholesterol drives the formation of a new phase. The liquid-ordered phase (3), as it is commonly called, is characterized by a lack of translational order in the membrane plane, like in a fluid phase, and by a strong conformational order among the lipid hydrocarbon chains, which in turn is reminiscent to the gel phase. Currently, it is thought that the liquid-ordered phase and cholesterol in particular play a prominent role in the functions of lipid rafts, which are strongly ordered membrane domains involved in a variety of cellular functions such as signal transduction and protein sorting; see below for additional discussion. However, despite their significant ordering, rafts are essentially fluid-like membrane domains, which highlight the importance of fluidity for membrane functions under physiologic conditions.

Figure 2. Lipid bilayer composed of DPPC lipids in two different phases. (a) Gel phase below the main transition temperature; (b) Fluid phase above the transition temperature. Water is not shown for clarity.
Length scales
To understand cellular functions that deal with single-molecule properties, we must achieve deeper knowledge about the structure and the molecular organization of lipid membranes on the nanometer scale. This knowledge is particularly important in the context of membrane proteins, because studies indicate that some membrane proteins favor certain specific lipids in their vicinity (9). Recent crystallographic studies of membrane protein structures have also revealed how lipids may be an integral part of the protein structure (15). However, as biological functions take place over a multitude of scales, one must also understand how structures and functions at larger scales emerge from corresponding ones at smaller scales. For example, membrane protein functions can be regulated by the (large-scale) elastic properties of membranes (16). Also, lipid aggregates such as micelles and vesicles are involved in intracellular transport and range in size from a few nanometers to micrometers; the size of a cell is typically about 30 micrometers. Therefore, no length scale would be specific to lipid structures.
Time scales
Time scales of dynamic processes in lipid systems are wide and range from picoseconds to hours or even months. Examples are discussed below. Here, we just refer to rotational motions as the fastest dynamical events and cell death as the slowest event. What is relevant to stress here is the lack of any specific time scale.
In summary, lipids play a prominent role in numerous cellular functions. Yet lipids and the structures composed of lipids are not characterized by any specific length or time scale. However, just like soft matter in general, there is one specific scale in common: the well defined energy scale given by the thermal energy kBT.
Lipids in Various Forms
The classification of lipids is largely arbitrary. It can be based on water solubility (hydration) or swelling of a lipid system at the air-water interface, for example. Here, we approach lipids in terms of increasing complexity and focus on those lipids that are found mostly in cells, see Fig. 1. A more thorough discussion of the topic is given by Hauser and Poupart (1) and Larson (4).
From fats to fatty acids and lipids
Fats are easy to recognize on the basis of our every-day experience. Usually, they are considered as frozen oils and used in cooking (17). The main component of a fat is a hydrocarbon moiety that is typically a long hydrocarbon chain, with a varying number of carbon atoms attached to each other through single (saturated) or double (unsaturated) bonds. Depending on the number of double bonds, the chains are called monounsaturated (one double bond), diunsaturated (two), or polyunsaturated (more than two). Fatty acids are obtained by attaching a carboxyl group at the end of a chain. Although hydrocarbons do not dissolve readily in water, fatty acids dissolve more easily because of the hydrophilic -COOH group. Yet fatty acids are not found free in the cell often. The rather few exceptions include the intercellular transport of fatty acids inside lipoproteins and the chemical reactions such as hydrolysis because of enzymes acting on lipids, in which a lipid is broken down into smaller pieces (one of them is a hydrocarbon chain). Fatty acids are linked more commonly to a chemical group that acts as the backbone of complex lipids. One of the most general groups is glycerol, which can form ester bonds in up to three positions. By doing so, one can form nonpolar lipids such as tri-acylglycerols (frequently also called triglycerides) with three ester bonds and di-acylglycerols with two ester linkages. Triglycerides are the major components of dietary fats and a common hydrophobic storage means of fat. They are also transported between cells via lipoproteins. Di-acylglycerols function as second messengers in signal transduction.
Another common backbone for fatty acids is sphingosine, which is a long-chain amine. Through an amide linkage, it is bound to a fatty acid, which forms the ceramide found often in skin and hair conditioners. As mentioned above, ceramide is also one of the signaling molecules involved in processes such as apoptosis, which is the programmed cell death.
Polar lipids
The above molecules are nonpolar and therefore hydrophobic. The polarity of lipids can be promoted by binding polar groups to glycerol- or sphingosine-based molecules. For example, the vacant -OH group in the glycerol moiety of di-acylglycerols can be linked to a polar group such as phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), phosphatidylglycerol (PG), or phosphatidylinositol (PI). PC and PE groups are neutral and zwitterionic, whereas PS, PG, and PI are anionic. Using glycerol as a backbone, one obtains glycerophospholipids, in which one of the above polar moieties usually is acting as the head group. The best known exception is cardi- olipin, in which two glycerophospholipids, which start from the phosphate group, are linked to one another through a glycerol group. Such a dimeric anionic lipid has usually four hydrocarbon chains. In a manner similar to glycerophospholipids, the free -OH group in ceramide (as the end group of sphingosine) can be linked to one of these head groups and one obtains sphingophospholipids. A common lipid of this group is sphingomyelin with a PC moiety as the head group.
One of the most complex classes of lipids in terms of their head group is glycolipids. Glycoglycerolipids are di-acylglycerols linked to a monosaccharide or a disaccharide such as galactose or lactose, respectively. In turn, glycosphingolipids are sphingolipids with a carbohydrate head group; the most common types are cerebrosides and gangliosides based on ceramide as the fundamental structure. Another subclass complementing the above ones is lipopolysaccharides. The complexity of glycolipids is caused not only by their structural diversity, but also by the stereo-chemistry that plays a major role in their properties. Carbohydrates in general are characterized by the lack of a well-defined structure-function relationship, which means that the details do matter and often turn out to be very important. For example, recent studies have shown that glycolipid bilayers with either a galactose or glucose head group yield substantially different membrane properties (18). Yet galactose and glucose differ only in chirality.
Nonetheless, the most fascinating and complex lipid type is sterols. They have a rigid steroid ring structure, which is a simple hydroxyl group as their polar head and a short flexible tail at the other end of the molecule. Of the many sterols found, two sterols exist whose significance in cells is above the others. Cholesterol is abundant in eukaryotic cells, and ergosterol is abundant in fungi. The reasons why nature has chosen these two specific sterols are not well understood. One is tempted to think that the properties of all sterols should be similar, because the structural differences between them are seemingly negligible. However, it has turned out that the details do matter. For example, adding just one double bond to the structure of cholesterol changes its membrane properties substantially (19). Consequently, cholesterol is the sterol in eukaryotic cells, and cholesterol esters are the form of cholesterol by which it is transported in lipoproteins.
Lipid composition of biological membranes and lipoproteins
The composition of lipids varies markedly in a cell, and biomembranes and lipoproteins provide crisp examples about the diversity of different lipids.
In the plasma membrane of animals (1), the amount of cholesterol is usually around 20-30 mol%. The rest of the lipids are mainly PC, PE, and sphingomyelin (SM) lipids, with smaller amounts of PS, PI, and glycolipids. These lipids are distributed asymmetrically across the membrane, because most cholesterol, PC, and glycolipids are located in the extracellular (outer) leaflet, whereas PS and PE lipids are located mainly in the intracellular (inner) monolayer. The lipid composition can be highly different in other organelles, however, as is the case in mitochondria (1), in which the mitochondrial membrane is composed of two (inner and outer) membranes. There, the amounts of cholesterol, SM, and PS are negligible; most lipids are PC and PE. The major difference compared with plasma membrane is the concentration of cardiolipins. They are actually found only in bacterial and in mitochondrial membranes, where their numbers are significant; even in mitochondria they are located mainly on the inner membrane.
In lipoproteins, one finds essentially the same types of lipids but with somewhat different concentrations. In order of decreasing numbers, low density lipoproteins contain (2) mainly cholesteryl esters, (unesterified) cholesterol, phosphatidylcholines, SM, triglycerides, and lyso-PC, and smaller numbers of PE, PI, and ceramide. In HDLs, one finds the same lipid types but in a different order, as HDLs are abundant in phospholipids and are complemented by cholesterol esters, cholesterol, and triglycerides.
Besides their head group, also other features differentiate lipids from one another. Among phospholipids, which is the main group of lipids in cells, about 10% are charged. Except for sphingosine, which is cationic, all other charged lipids are anionic. What is even more striking is the diversity of unsaturation and chain length. The number of double bonds per hydrocarbon chain ranges from zero to six, and the chain length ranges typically from 14 to 22 carbons per chain. Usually the sn-2 chain in a glycerophospholipid is unsaturated, whereas the sn-1 chain is saturated, and these two chains may be asymmetric in terms of chain length. Furthermore, in addition to ester lipids, ether lipids such as plasmalogens exist, in which the hydrocarbon chain is linked to glycerol by an ether bond instead of an ester bond. For example, ether lipids act in cell signaling.
The above examples highlight the extraordinary diversity of lipids. On a cellular level, any single membrane may contain more than 100 different lipid species, each assumed to have some particular function. Because nature always has a reason, a reason must exist for having all these lipids. Although we do not understand the roles of all lipids at the moment, it is rather obvious that each one participates in some specialized functions.
Methods Commonly Applied to Lipid Systems
An extensive toolbox is often applied to lipid systems. Here, let us discuss some of the most important techniques, considering both experimental as well as computational ones. Although the description here is inevitably brief, it hopefully provides some flavor to the techniques in question.
Experimental techniques
One of the most versatile methods for studies of lipid systems is NMR (13, 20). In NMR, an applied magnetic field is used together with an alternating electromagnetic field to probe changes in molecular alignment. NMR can provide detailed information on the topology, the dynamics, and the structure of molecules. In the context of lipids, it allows determination of the orientation of a lipid or a group of atoms in a lipid, such as the head group region or a single C-H bond in a hydrocarbon chain. The latter in particular is often used to determine the ordering of lipid hydrocarbon chains by selective deuteration of acyl chains, as it provides one with a solid understanding of fluidity inside a membrane. As for dynamics, NMR is commonly employed to explore the rotational motion of atom groups in lipids and the diffusion of lipids in the plane of a membrane.
Small angle X-ray scattering (SAXS) (13, 21) is used increasingly in biological sciences to determine dynamic structures of various molecular systems. The X-ray sources with high intensity allow the observation of weak scattering features that are associated with the internal structures of molecules studied. In the context of lipids, SAXS is often used to measure lipid system structures and their phase behavior.
Differential scanning calorimetry (DSC) (22) is based on measuring changes in heat capacity because the temperature of a system is varied monotonically. As heat capacity is a thermodynamic response function, it is expected to exhibit critical behavior close to a phase transition boundary, which in turn can be detected by DSC. Consequently, DSC is a common way to detect phase behavior.
Considering techniques that allow the imaging of lipid surfaces, scanning probe microscopes such as the atomic force microscope (AFM) (13, 23) have become very appealing. The AFM allows measurements of native lipid samples under physiologic-like conditions and while biological processes are at work. It is hence often used to determine lipid membrane structures, structural defects in membranes, domain formation, and even the behavior of lipid rafts with high nanometer-scale lateral resolution.
Another appealing technique used often to image lipid structures is fluorescence microscopy and imaging (24). By using fluorescent probes attached covalently to lipid molecules, the fluorescence microscopy provides a wealth of spatially resolved information of individual lipid aggregates instead of averaging over a large number of them, see Fig. 3. Hence, fluorescent probes provide detailed information of structure as well as dynamics, and this information is often exploited to investigate phenomena such as trafficking and membrane dynamics in the spirit of single-particle tracking and single-molecule detection (14, 25). The downside is that probes inevitably perturb the system (26, 27), thus their use and the interpretation of the results warrant particular care.

Figure 3. Liposomes of varying lipid and protein composition visualized using fluorescence microscopy with different fluorescent probes. The typical size of a liposome is approximately 40 micrometers. Pictures are by courtesy of Luis Bagatolli and are available at the MEMPHYS website (Science In Your Eyes): www.memphys.sdu.dk.
Computational modeling
Unlike simulations of proteins and protein complexes, modeling and simulations of lipid systems is relatively “easy” in the sense that lipid molecules and (smallest) lipid aggregates are reasonably small, and the time scales related to many processes that take place in lipid systems are of the order of nanoseconds. Consequently, even atomistic modeling of lipid aggregates is feasible for reasonably complex systems. Here, we discuss briefly the three main levels of modeling associated with lipids.
Quantum-mechanical (QM) modeling
QM methods (28) are crucial in studies of processes in which one must account for electronic degrees of freedom, such as the action of sphingomyelinase acting on sphingomyelin. Such enzymes hydrolyze lipids, which cuts them into pieces. To describe the hydrolysis process fully, one should treat the action center in a QM manner. The problem is that QM techniques are feasible only to small scales, typical system sizes are a few hundred atoms and time scales range up to tens of picoseconds. Obviously, these scales are short compared with most biologically relevant processes. Consequently, QM techniques are often bridged to classical simulations in a manner in which the region of interest (such as the reaction center) is described in a QM fashion, whereas the rest of the system follows classical equations of motion using molecular mechanics (MM) force fields. Such QM/MM methods have gained increasing popularity in the field, but their wider use is limited to difficulties of treating the interface accurately between QM and classical regions. However, recent studies have shown that the QM/MM techniques work well, and the prospects for broader applications are promising (29).
Classical atomistic simulations
The most popular technique to deal with lipid systems has by far been classical molecular dynamics (MD) (28, 30). In MD, all interactions are classic, and the time evolution of the system is described by integrating Newton’s equations of motion. The particles can represent atoms or clusters of atoms; the most typical choice is the full-atom description in which all atoms including hydrogens are described explicitly, and the united-atom description in which each methyl and methylene group is described by a single particle. The particle-particle interactions are usually determined from QM calculations and tuned even more in an iterative manner by fitting system properties to experiments until simulation results and experimental data match sufficiently well. Usually, the largest system sizes are hundreds of thousands of atoms over hundreds of nanoseconds, which renders possible studies of various relevant processes. Examples are presented below. However, considering a practical example for a lipid membrane, the largest systems considered to date have been about 20 nm x 20 nm in size in the membrane plane, which is rather small compared with typical membrane domain sizes. This finding implies that atomistic MD simulations currently are not the method of choice for many-component membrane systems, in which time scales of mixing and domain formation are far beyond the limits of atomistic simulations. To reach such scales, efficient ways are needed to treat the dynamics or the models that are simpler than the atomistic ones. The first idea can be conducted by, for example, Monte Carlo simulations (28, 30, 31). Instead of integrating the equations of motion in a deterministic manner, one employs random noise to evolve the system from one configuration to another in a random fashion. Despite the fact that Monte Carlo simulations (usually conducted with the Metropolis scheme) do not yield natural dynamics, they gauge equilibrium properties that are not functions of time. What is more, the Monte Carlo approach allows the use of nonphysical moves, which usually provides a major speed-up, hence facilitating studies of very large systems. The second idea to deal with simplified (coarse grained) models is more common, however, because it allows one to consider dynamic quantities and nonequilibrium properties.
Coarse-grained models
The key idea of coarse-grained (CG) models (32, 33) is to get rid of all details that are not relevant for the properties one is interested in. Replacing CH2 groups by a united-atom particle can be considered coarse graining. In a similar fashion, one can replace several (say, four) methyl groups in a lipid hydrocarbon chain by a coarse-grained particle. Because the number of interacting beads is reduced, the computational burden decreases as well, which allows one to consider larger systems and time scales. This strategy is used more and more often in simulations of lipid systems, and the results have been very encouraging. The main challenges associated with CG models are the choice of the coarse-grained molecular description, for which one can employ systematic techniques such as self-organizing maps (34) together with plain intuition, and the choice of the interactions used in the CG model. For the latter issue, several approaches have been proposed. Perhaps the most promising approach is presented by Marrink et al. (35, 36), who used thermodynamic quantities such as solvation free energies to determine interaction strengths for different molecular groups. Another means to improve coarse graining is to get rid of the solvent (37, 38). This solvent-free approach can provide a major speed-up for dynamics, although it also has obvious limitations because of the absence of full hydrodynamics.
Structure of Lipid Membranes and Importance of Cholesterol
One of the main functions of lipids is to serve as the main structural components of cell membranes (7). Membranes resemble thin elastic sheets with a total thickness of about 5 nm. The membrane is composed of two lipid monolayers. The lipids in a membrane typically include two nonpolar and hydrophobic (water hating) acyl chains connected to one another close to the head group, which in turn is usually polar and hydrophilic (water loving) and therefore can form hydrogen bonds with neighboring water molecules. This “schizophrenic” nature of lipid molecules causes them to self-assemble as closed objects such as liposomes, such that the head groups face water molecules while the hydrophobic hydrocarbon chains are protected from the water phase.
The essential structure of membranes is captured by the single-component lipid bilayer shown in Fig. 2. Although this view is a highly simplified description of an actual biological membrane, it readily demonstrates the importance of lipids on membrane structure and dynamics. Native biological membranes found in living systems are composed of lipids in terms of a lipid bilayer; although in those cases, it is not a single-component but rather is a many-component membrane that consists of hundreds of different types of lipids that differ from one another in several ways such as size, unsaturation level, chemical composition in the polar head group region, number of hydrocarbon chains, and charge. The bilayer in native membranes also acts as a soft fluid-like environment for integral and peripheral proteins embedded in or attached peripherally to the membrane. In addition, the membrane proteins are involved in a dynamic rubber-like network known as the cy- toskeleton attached to the inner surface of the membrane. The cytoskeleton moves the cell, gives even more rigidity to the membrane, and also allows the membrane to adjust its shape to varying nonspherical shapes. Moving on, also the outer leaflet of the membrane is covered by a network, which in this case is made of carbohydrates. The glycocalyx network, as it is called, is involved in cell-cell recognition and adhesion to other cells, among other functions.
In eukaryotic cells, one of the most important, or even the most important, lipid is cholesterol. Cholesterol constitutes about 30-40 mol% of the plasma membrane, and in the ocular lens membrane, its amount can be as large as about 80 mol%. Therefore, the role of cholesterol deserves the particular attention discussed below.
Influence of cholesterol
Cholesterol affects a large variety of membrane properties in animal cells (39). It is involved in modifying dynamical membrane properties by reducing passive permeation, slowing down the lateral diffusion of molecules in fluid-like membranes, and speeding up diffusion in gel-phase membranes. It also affects bilayer properties by condensing the bilayer, which changes its elastic properties and promotes the order of phospholipid acyl chains in the hydrophobic membrane core. In this manner, cholesterol develops the formation of the liquid-ordered phase (3), which is characterized by significant conformational order in the lipid hydrocarbon chain region and the absence of translational long-range order in the membrane plane. Through the formation of the liquid-ordered phase, cholesterol governs membrane fluidity and is associated with membrane domain formation, in particular the formation of lipid rafts (8). The role of cholesterol in rafts perhaps best underlines the biological importance of cholesterol, see below for discussion. What is more, cholesterol seems to have a unique structure-function relationship because many cells do not do well without cholesterol. For example, desmosterol, which differs from cholesterol only by one double bond in the short hydrocarbon tail, cannot substitute for cholesterol (19).
The ordering capability of cholesterol is illustrated by Fig. 4, which shows the effect of increasing cholesterol concentration on the ordering of lipid hydrocarbon chains in a fluid DPPC bilayer (39). In the absence of cholesterol, the NMR order parameter, SCD, indicates reasonable conformational order close to the membrane-water interface, and monotonically decreasing order toward membrane center. For increasing cholesterol concentration, the conformational order increases significantly. For the largest cholesterol concentration of 50 mol%, the order parameter is close to its maximal value of 0.5, in which case the chain would be in a full-trans conformation standing along the membrane normal direction. The enhanced ordering of hydrocarbon chains because of increasing cholesterol concentration is coupled to stronger packing, which in turn reduces the amount of free volume and changes the shape and size distributions of free volume pockets inside the membrane (40). It is readily clear why cholesterol plays such a strong role in many dynamic membrane processes. Yet the biological relevance of cholesterol in cells is related largely to lipid rafts.

Figure 4. Order parameter profiles (39) for the lipid hydrocarbon (a) sn-1 and (b) sn-2 chains in a binary membrane mixture of DPPC and cholesterol. The cholesterol concentrations are 0 mol% (open circle), 5 mol% (full circle), 12 mol% (open square), 20 mol% (full square), 30 mol% (open diamond), and 50 mol% (full diamond), and the index n for carbons in a hydrocarbon chain increases toward the membrane center.
In 1997, Simons and Ikonen (8) proposed that strongly ordered membrane domains rich in cholesterol and sphingolipids would be involved in a variety of cellular processes such as signal transduction, protein sorting, and programmed cell death. Ever since, the research that focuses on lipid rafts (see Fig. 5), as they are commonly called, has been very intense (41). Although the studies have demonstrated the role of rafts in numerous processes, the definition and the structure of rafts are still under debate. The uncertainties regarding the structure of rafts is largely caused by the small length and time scales, because the sizes of rafts seem to range from a few nanometers to hundreds of nanometers, and the time scales are short because of the transient nature of rafts. These conditions pose a challenge to gauge raft systems through experiments, thus the precise understanding of the structure within rafts has remained limited.

Figure 5. Snapshot of a lipid raft system studied through atomistic molecular dynamics simulations (42). Water is shown at the top and at the bottom in light color, while the membrane is in the middle of the figure. In the bilayer, rigid cholesterol molecules are shown in light grey, POPC in dark grey, and sphingomyelin in intermediate grey.
Recent atom-scale simulations have complemented experiments and have shed some light on the matter (42). As Fig. 6 shows, cholesterol strongly orders the lipids in its vicinity, which condenses the membrane. The interplay of cholesterol with sphingolipids develops domains that are particularly highly packed, which in turn slows down the lateral diffusion rate substantially compared with other membranes with large amounts of cholesterol. Cholesterol plays a role in the lateral pressure profile that acts on proteins embedded in a membrane. Recent simulations have shown that lipid rafts have distinctly different pressure profiles compared with other membrane systems, and that the contribution of the pressure profile for the free energy barrier for membrane protein activation (using a model of MscL as an example) can be considerable compared with the total free energy barrier (42). All together, the results highlight the distinct nature of rafts and the importance of cholesterol in membranes overall. However, whereas the understanding of rafts and their role in cellular functions has made considerable progress during the last decade, major gaps remain to be covered by a combination of novel experimental and theoretical efforts.

Figure 6. Results for lipid raft systems with varying amounts of POPC (palmitoyl-oleoyl-phosphatidylcholine), SM, and cholesterol (42), which shows plots for one leaflet from above. The top row for the system SA is a 1:1:1 mixture of POPC, SM, and cholesterol. The middle row shows data for SB (2:1:1 for POPC:SM:cholesterol), and the bottom row shows data for SC (62:1:1 for POPC:SM:cholesterol). The first column on the left shows data for the acyl chain order parameter (SCD) averaged over selected carbons in POPC and SM chains. The second and third columns depict the in-plane electron densities of cholesterol and the selected chain carbons, respectively.
The dynamics of lipids and lipid aggregates is driven mainly by thermal fluctuations through kBT. This finding is truly fascinating because it implies that nature uses random walks in essentially all dynamic processes: The diffusion of lipids in membranes is a random walk, the growth of microtubulin follows a random-walk line pattern, and the (passive) diffusion inside a cell overall is caused by the thermal forces that act on lipid systems (43). To complement thermal diffusion, active dynamic processes occur in which the chemical energy contained in ATP is converted into mechanical work done by motor proteins. These active processes are also involved partly in the dynamics of lipids, such as in the one-dimensional diffusion of motor proteins that drag cellular cargo inside a lipid droplet along a tubulin. However, we focus here on the passive dynamic processes driven by thermal energy.
Considering dynamic processes in fluid membranes as an example and starting from the fastest dynamical processes, one may first consider rotational diffusion of individual carbon- hydrogen bonds in CH2 groups in lipid hydrocarbon chains. The time scale of these rotational motions is on the order of picoseconds. The rotational motion of whole lipids around their principal axes of rotation is a slower process and usually takes place over a scale of nanoseconds. Lateral diffusion, in turn, involves diffusion of matter and hence longer time scales. This time scale is characterized by the diffusion length λ = (2d D T)1/2, where d is the dimensionality, D is the diffusion coefficient (~10-7 cm2/s in fluid membranes), and T is the time scale considered for diffusion. On average, a lipid in a fluid membrane diffuses over a distance of its own size (~0.8 nm) in about 15-20 ns. The mixing of different lipid components and domain formation requires longer times and depends on the length scale. Assuming a membrane domain with a radius of 100 nm, an individual lipid would cross this length on average in about 250 microseconds. In a similar manner, assuming a roughly constant diffusion coefficient in all regions in a membrane, the average time scale for diffusion from one point in a plasma membrane (surrounding the cell) to the other side of the cell would be about a minute. One minute-is this a lot? No. It is vanishingly small compared with the time scales of many cellular functions or, say, protein folding. Diffusion over cellular scales along the membrane plane is an efficient means to transport molecules, and for a cell this comes for free. No ATP is wasted. Instead, thermal fluctuations drive the motion.
All dynamic events are not so rapid, however. Lipid flip-flop, in which a lipid translocates from one leaflet to another in a membrane, is usually a profoundly slow process and occurs in a time scale of minutes or even hours. As for the longest time scale associated with biological lipid systems, the typical life time of a cell ranges from a few days to several months, which depends on the tissue type.
The above estimates are for fluid-like systems. In gel-like systems with features of frozen order, the time scales are much longer. For example, the lateral diffusion coefficient in a gel-like one-component membrane is about 10-16-10-10 cm2/s (43), whereas in a fluid membrane it is usually ~10-7 cm2/s. In a similar manner, the diffusion of matter inside lipid droplets is a much slower process compared with lipid interfaces caused by entanglement effects, as the situation is largely similar to a polymer melt. This effect is the case inside LDL. It has been estimated that the diffusion coefficient for cholesterol esters inside LDL particles is roughly 10-9 cm2/s (44) and is intermediate to diffusion in fluid- and gel-like membranes.
Overall, the mechanisms associated with dynamic processes in lipid systems are complex and are understood rather poorly, although the combination of experiments and computer simulations has improved the situation recently. As an example, let us consider a more concrete situation, the formation of pores in a cell membrane and its significance for cellular functions.
Pore formation in lipid membranes
Transient water pores in cellular membranes are involved in several relevant processes, such as maintenance of osmotic balance, drug and antibody delivery into cells, and ion transport across the membrane. Understanding ion transport across membranes is especially important, because membranes strive to maintain a cationic electrochemical gradient used for ATP synthesis. Yet, ions leak through lipid membranes, and understanding the mechanisms associated with ion leakage would allow one to control membrane properties better in related applications.
Figure 7 illustrates the complexity of transient pore formation (45) under conditions that closely resemble physiologic conditions. The initial ion concentration imbalance across the membrane develops a strong local electric field that induces the formation of a pore. The pore formation starts with the creation of a single water defect in terms of a chain of water molecules, which spans the entire membrane. The defect then expands within less than 1 ns through redistribution of lipid head groups close to the defect. Despite its transient nature, the pore is relatively stable and facilitates the transport of ions through the pore along the ion concentration gradient, which in turn leads to a reduction of the transmembrane electric field. When the ion concentration imbalance has reduced below some threshold value, the pore becomes unstable and eventually closes. Usually, the lifetime of the pore ranges from about 50 ns to a few hundred ns, highlighting the very rapid nature of the process.

Figure 7. Snapshots of pore formation and the resulting ion leakage across a lipid membrane (45). The plots (a)-(f) demonstrate the process at different times after the initial electric field has been established: (a) 20 ps; (b) 450 ps; (c) 1000 ps; (d) 1070 ps; (e) 9180 ps; (f) 60 ns. The membrane is not shown for clarity, whereas water is shown in intermediate grey and Na+ ions as light spheres.
The pore formation mechanism presented in Fig. 7 (45) is appealing for many reasons. It illustrates the significance of thermal fluctuations, because the pore is indeed induced by fluctuations in spontaneous salt ion concentrations in the vicinity of the membrane. Furthermore, the pore mediated ion leakage mechanism is very rapid, and it occurs in a collective manner through redistribution and diffusion of lipids around the pore. Also, recent data indicate that the pores also mediate flip-flop events across a membrane (46), which provides one plausible mechanism for lipid translocation, which in turn is of central importance in processes such as programmed cell death. Other dynamic processes in lipid systems are expected to be equally complex, which highlights the importance to understand the interplay between thermal fluctuations, physiologic conditions, and collective phenomena.
Influence of Probes
Often, experimental studies of lipid systems are based on spectroscopic approaches, which in turn frequently employ probes for enhancement of sensitivity and resolution. For example, in NMR, hydrogen atoms of lipids are replaced with deuterium, and in fluorescence spectroscopy and imaging, native lipid molecules are replaced with lipids in which one of the hydrocarbon chains is linked covalently to a fluorescent marker such as pyrene or diphenylhexatriene. Fluorescent markers allow one to follow numerous cellular processes in real time, such as intracellular trafficking of molecules and formation of domains within a biomembrane, see Fig. 3. The downside is that the probes tend to perturb their environment and affect the thermodynamic state of the system. Experiments have shown, for example, that probes may change the main transition temperature of a lipid membrane, and that the dynamics of probes may deviate considerably from the dynamics of corresponding native molecules (see discussion in Reference 27). Therefore, we wish to pose several questions. What is the range of perturbations induced by the probe? How significant are these perturbations actually?
Figure 8 shows a plot of a fluid-like DPPC bilayer, in which a small fraction of the lipids are replaced by a pyrene-containing PyrPC probe, see Fig. 1 (26). The study has demonstrated that the perturbations in the vicinity of the probe are substantial, as the conformational order parameter (SCD) of lipid hydrocarbon chains close to the probe may change as much as about 100%. However, what is also found is the short range of perturbations, because the perturbations are negligible beyond a distance of about 1.5 nm. In practice, this finding implies that about 20-30 lipids around the probe are affected by the marker, but the global properties averaged across the membrane are affected only little.
This brief example highlights the possible problems associated with using fluorescent probes and antibodies and with interpreting the results obtained through probes. However, fluorescent labels are one of the most appealing means to follow a variety of intriguing dynamic processes in biological matter. Although there is a reason to be cautious, there is even more reason to develop better probes that mimic the properties of native molecules as closely as possible.
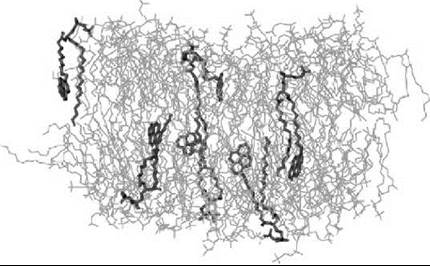
Figure 8. Snapshot of a DPPC bilayer with a small concentration of DPPCs replaced with PyrPC probes, see Fig. 1 (26). One finds PyrPC probes (shown in dark grey) to penetrate (interdigitate) significantly into the opposing leaflet, thus causing perturbations in both membrane monolayers. Water is not shown for clarity.
Acknowledgments
We wish to thank our collaborators whose contribution for the results presented here has been crucial. Additionally, Jarmila Repakova, Emppu Salonen, Tomasz Rog, Ole Mouritsen, and Luis Bagatolli are thanked for help with illustrations.
References
1. Hauser H, Poupart G. Lipid structure. In: The Structure of Biological Membranes. Yeagle Philip L, ed. 2005. CRC Press, Boca Raton, FL.
2. Hevonoja T, Pentikainen MO, Hyvonen MT, Kovanen PT, Ala-Korpela M. Structure of low-density lipoprotein (LDL) particles: basis for understanding molecular changes in modified LDL. Biochim. Biophys. Acta 2000; 1488:189-210.
3. Mouritsen OG. Life - As a matter of fat: the emerging science of 19. lipidomics. 2005. Springer-Verlag, Berlin.
4. Larsson K. Lipids - Molecular Organization, Physical Functions and Technical Applications. 1994. The Oily Press, Dundee, Scotland.
5. Ball P. The Self-made Tapestry. 1999. Oxford University Press, Oxford.
6. Jones MN, Chapman D. Micelles, Monolayers, and Biomembranes. 1995. John Wiley & Sons, New York.
7. Yeagle PL, ed. The Structure of Biological Membranes, 2nd edition. 2005. CRC Press, Boca Raton, FL.
8. Simons K, Ikonen E. Functional rafts in cell membranes. Nature 1997; 387:569-572.
9. Soubias O, Teague WE, Gawrisch K. Evidence for specificity in lipid-rhodopsin interactions. J. Biol. Chem. 2006; 281:33233-33241.
10. Kinnunen PKJ, Holopainen JM. Sphingomyelinase activity of LDL: A link between atherosclerosis, ceramide, and apoptosis? Trends Cardiovasc. Med. 2002; 12:37-42.
11. Veldhuizen EJA, Haagsman HP. Role of pulmonary surfactant components in surface film formation and dynamics. Biochim. Biophys. Acta 2000; 1467:255-270.
12. Porter CJH, Trevaskis, NL, Charman, WN. Lipids and lipid-based formulations: Optimizing the oral delivery of lipophilic drugs. Nat. Rev. Drug Discov. 2007; 6:231-248.
13. Katsaras J, Gutberlet T, eds. Lipid Bilayers: Structure and Interactions. 2001. Springer-Verlag, Berlin.
14. Serdyuk IN, Zaccai NR, Zaccai J. Methods in Molecular Biophysics: Structure, Dynamics, Function. 2007. Cambridge University Press, Cambridge, UK.
15. Fyfe PK, Hughes AV, Heathcote P, Jones, MR. Proteins, chlorophylis and lipids: X-ray analysis of a three-way relationship. Trends Plant Sci. 2005; 10:275-282.
16. Lundbaek JA. Regulation of membrane protein function by lipid bilayer elasticity — a single molecule technology to measure the bilayer properties experienced by an embedded protein. J. Phys. Condens. Matter 2006; 18:1305-1344.
17. Barham P. The Science of Cooking. 2001. Springer-Verlag, Berlin.
18. Rog T, Vattulainen I, Bunker A, Karttunen M. Glycolipid membranes through atomistic simulations: effect of glucose and galactose head groups on lipid bilayer properties. J. Phys. Chem. B 2007; 111:10146-10154.
19. Vainio S, Jansen M, Koivusalo M, Rog T, Karttunen M, Vat- tulainen I, Ikonen E. Significance of sterol structural specificity— desmosterol cannot replace cholesterol in lipid rafts. J. Biol. Chem. 2006; 281:348-355.
20. Hounsell EF. NMR of carbohydrates, lipids and membranes. In: Specialist Periodical Reports on NMR, Vol. 34. 2005. The Royal Society of Chemistry, London.
21. Nolting B. Methods in Modern Biophysics. 2005. Springer-Verlag, Berlin.
22. Hsueh YW, Gilbert K, Trandum C, Zuckermann M, Thewalt J. The effect of ergosterol on dipalmitoylphosphatidylcholine bilayers: a deuterium NMR and calorimetric study. Biophys. J. 2005; 88:1799-1808.
23. Alessandrini A, Facci P. AFM: a versatile tool in biophysics. Meas. Sci. Technol. 2005; 16:65-92.
24. Bagatolli LA. To see or not to see: lateral organization of biological membranes and fluorescence microscopy. Biochim. Biophys. Acta 2006; 1758:1541-1556.
25. Saxton MJ, Jacobson K. Single-particle tracking: applications to membrane dynamics. Annu. Rev. Biophys. Biomol. Struct. 1997; 26:373-399.
26. Repakova J, Holopainen JM, Karttunen M, Vattulainen I. Influence of pyrene-labeling on fluid lipid membranes. J. Phys. Chem. B 2007; 110:15403-15410.
27. Repakova J, Holopainen JM, Morrow MR, McDonald MC, Capkova P, Vattulainen I. Influence of DPH on the structure and dynamics of a DPPC bilayer. Biophys. J. 2005; 88:3398-3410.
28. Leach A. Molecular Modelling: Principles and Applications, 2nd edition. 2001. Prentice Hall, Englewood Cliffs, NJ.
29. Senn HM, Thiel W. QM/MM methods for biological systems. Top. Curr. Chem. 2007; 268:173-290.
30. Schlick T. Molecular Modeling and Simulation: An Interdisciplinary Guide. 2002. Springer-Verlag, New York.
31. Chiu SW, Jakobsson E, Subramaniam S, Scott HL. Combined Monte Carlo and molecular dynamics simulation of fully hydrated dioleyl and palmitoyl-oleyl phosphatidylcholine lipid bilayers. Biophys. J. 1999; 77:2462-2469.
32. Karttunen M, Vattulainen I, Lukkarinen A, eds. Novel Methods in Soft Matter Simulations. 2004. Springer-Verlag, Berlin.
33. Chu JW, Izveko S, Voth GA. The multiscale challenge for biomolecular systems: coarse-grained modeling. Mol. Simulat. 2006; 32:211-218.
34. Murtola T, Falck E, Vattulainen I. Conformational analysis of lipid molecules by self-organizing maps. J. Chem. Phys. 2007; 126:054707.
35. Marrink SJ, de Vries AH, Mark AE. Coarse grained model for semiquantitative lipid simulations. J. Phys. Chem. B 2004; 108:750-760.
36. Marrink SJ, Risselada HJ, Yefimov S, Tieleman DP, de Vries A. The MARTINI force field: Coarse grained model for biomolecular simulations. J. Phys. Chem. B 2007; 111:7812-7824.
37. Cooke IR, Deserno M. Solvent-free model for self-assembling fluid bilayer membranes: stabilization of the fluid phase based on broad attractive tail potentials. J. Chem. Phys. 2005; 123:224710.
38. Brannigan G, Brown FLH. Solvent-free simulations of fluid membrane bilayers. J. Chem. Phys. 2004; 120:1059-1071.
39. Falck E, Patra M, Hyvonen MT, Karttunen M, Vattulainen I. Lessons of slicing membranes: interplay of packing, free area, and lateral diffusion in phospholipids/cholesterol bilayers. Biophys. J. 2004; 87:1076-1091.
40. Falck E, Patra M, Karttunen M, Hyvonen MT, Vattulainen I. Impact of cholesterol on voids in phospholipid membranes. J. Chem. Phys. 2004; 121:12676-12689.
41. Jacobson K, Mouritsen OG, Anderson RGW. Lipid rafts: at a crossroad between cell biology and physics. Nat. Cell Biol. 2007; 9:7-14.
42. Niemela P, Ollila S, Hyvonen MT, Karttunen M, Vattulainen I. Assessing the nature of lipid rafts. PLoS Comput. Biol. 2007; 3:304-312.
43. Vattulainen I, Mouritsen OG. Diffusion in membranes. In: Diffusion in Condensed Matter: Methods, Materials, Models. Heitjans P, Karger J, eds. 2005. Springer-Verlag, Berlin.
44. Heikela M, Vattulainen I, Hyvonen MT. Atomistic simulation studies of cholesteryl oleates: model for the core of lipoprotein particles. Biophys. J. 2006; 90:2247-2257.
45. Gurtovenko AA, Vattulainen I. Pore formation coupled to ion transport through lipid membranes as induced by transmembrane ionic charge imbalance: atomistic molecular dynamics study. J. Am. Chem. Soc. 2005; 127:17570-17571.
46. Gurtovenko A, Vattulainen I. Molecular mechanism for lipid flip-flops. J. Phys. Chem. B 2007; 111:13554-13559.
Further Reading
Bloom M, Evans E, Mouritsen OG. Physical properties of the fluid lipid-bilayer component of cell membranes: a perspective. Q. Rev. Biophys. 1991; 24:293-397.
Cevc G, Marsh D. Phospholipid Bilayers: Physical Principles and Models. 1987. Wiley, New York.
Finegold L, ed. Cholesterol in Membrane Models. 1993. CRC Press, Boca Raton, FL.
Lipowsky R, Sackmann E, eds. Structure and Dynamics of Membranes: From Cells to Vesicles. 1995. Elsevier, Amsterdam, The Netherlands.
Merz KM Jr, Roux B, eds. Biological Membranes: A Molecular Perspective from Computation and Experiment. 1996. Birkhauser, Boston, MA.
See Also
Passive Diffusion Across Membranes
Lipid Domains, Chemistry of
Membranes, Fluidity of
Lipids, Chemical Diversity of