CHEMICAL BIOLOGY
Carbohydrate-Carbohydrate Interactions
Nicole Seah and Amit Basu,, Brown University,, Providence,, Rhode Island
doi: 10.1002/9780470048672.wecb199
Carbohydrate-carbohydrate interactions have been suggested as mediators of cell adhesion and aggregation. Studies of four different interactions— sponge cell aggregation, embryo and myelin compaction, and melanoma cell adhesion—have provided insights into the role of the saccharides in these events. The biological context of these associations as well as the results of experiments using biophysical and chemical model systems are described.
Cell-surface glycans in the form of glycolipids, glycoproteins, proteoglycans, and glycocalyx polysaccharides play an important role in biological recognition processes. Although most known receptors for cell-surface carbohydrates are proteins (i.e., lectins), several studies over the past few decades have clearly established that carbohydrates can function as binding partners for each other. These investigations of carbohydrate-carbohydrate interactions (CCIs) are broadening our view about the function of cell-surface glycans and may lead to a more thorough understanding of the principles of carbohydrate recognition. Although noncovalent interactions between individual residues of polysaccharides such as glycosaminoglycans and hyaluronic acid have long been recognized (1), the role of CCIs in cell adhesion and signaling is not fully understood. Some types of CCIs, such as those involved in sponge cell adhesion, are thought to have primarily structural roles. In other instances, such as melanoma cell adhesion, the CCI is believed to initiate signal transduction.
Chemical structures of carbohydrate epitopes from four well-studied CCIs are shown in Fig. 1. CCIs can occur via homotopic self-recognition, or they can be heterotopic. A homotopic interaction of the sulfated disaccharide 1 mediates sponge cell adhesion, whereas self-recognition of the Lewis x trisaccharide 2 has been identified in embryonic development. Divalent cations are required for these two homotopic CCIs. The interaction between the glycosphingolipid GM3 (3) and the glycolipids LacCer (4) or Gg3 (5) mediate the adhesion of melanoma cells to lymphoma or endothelial cells and initiate signal transduction. Carbohydrate epitopes involved in CCIs can also be as small as a monosaccharide, as is the case with the glycolipids GalCer (6) and cerebroside sulfate (CBS, 7) found in the myelin sheath. These heterotopic CCIs also use calcium, but the metal does not seem to be essential for association. Other carbohydrates that engage in CCIs have been reported for the aggregation of human embryonal carcinoma cells and trout sperm fertilization (2, 3).
All structures shown in Fig. 1 are involved in a trans-interaction, which requires that sections of the apposing cell membranes be brought in close proximity so that membrane-bound carbohydrates can interact with each other. This type of cell junction has been referred to as a glycosynapse, an analogy to immunolgical and neurological synapses 4. The existence and characterization of glycosynapses and the elucidation of their mechanisms of action remain important challenges for the field of cell-surface carbohydrate recognition. Alternatively, preliminary evidence has hinted at the occurrence of CCIs between carbohydrates that are present on the same membrane; i.e., interactions in cis. In these cases, a glycolipid has been shown to perturb the function of an N-glycosylated membrane-bound protein (5-7). Although the perturbation may be a result of a CCI between the glycolipid and the N-glycan, a specific and conclusive demonstration of a CCI in these systems awaits further studies.
The following sections provide a biological context for each of the four well-characterized CCIs shown in Fig. 1 and describe in greater detail the results of studies using a variety of chemical model systems and biophysical tools. It is notable that despite the very different contexts for these interactions (e.g., glycolipid vs. proteoglycan), in each case the minimal carbohydrate residues alone are necessary and sufficient for association, as long as the sugars are presented in a multivalent format. This ability to reproduce CCIs in model systems (e.g., glycosylated polymers, surfaces, proteins, vesicles, etc.) has greatly facilitated studies of this phenomenon. Although biomimetic models necessarily do not capture all components of a CCI present in the cellular context, they provide insights into carbohydrate molecular recognition. Additionally, these model systems offer tools that can subsequently be used to study the biological functions of CCIs in their more complex native environments.

Figure 1. Glycan structures that participate in carbohydrate-carbohydrate interactions.
CCI in Sponge Cell Adhesion
Biological Context
One of the first examples of a CCI was identified in various sponge species, where interactions between cell-surface proteoglycans mediate the self-association of sponge cells (8). Sponges that have been dissociated into their component cells are capable of reassociating in a species-specific manner. This phenomenon has been extensively studied using the marine sponge Microciona prolifera as a model system. The selective self-association is mediated by a proteoglycan referred to as the Microciona aggregation factor (MAF). MAF contains an N-linked sulfated glycan (g200) composed of fucose, glucuronic acid, mannose, galactose, and N-acetyl glucosamine (9). MAF extracted from sponge cells does not bind to other sponge cells until it is rendered multivalent by glutaraldehyde cross-linking (10). MAF-mediated cell aggregation can be inhibited by several antibodies that bind to specific carbohydrate epitopes within the glycan. The Block 1 antibody reactive epitope is a carbohydrate containing a novel pyruvate ketal (11) A second epitope, reactive with the Block 2 antibody, was identified as GlcNAc(3SO3)β1-3Fuc (1). The identity of these oligosaccharide epitopes was determined by degradation of the polysaccharide followed by nuclear magnetic resonance (NMR) and microscopic (MS) analysis. Other oligosaccharide components of proteoglycan aggregation factors from additional sponge species have also been identified (12).
Model Studies
Early studies of sponge cell aggregation demonstrated that CCIs were still observed when the glycans were removed from their native context. A striking visual example of the species specificity of CCI is evident in the aggregation of proteoglycan-attached microbeads, shown in Fig. 2 (13). Figure 2a shows three different sponges, each with a distinct coloration. Adhesion factors from the sponges were removed and covalently attached to beads color-coded pink, yellow, or white for each sponge. When the three beads were mixed together in the presence of calcium, they self-sorted into singly colored clusters based on the origin of the glycans on each bead (Fig. 2b). In contrast, if the pink, yellow, and white beads were all coated with the same glycan, no color sorting was observed (Fig. 2c). These glycan-coated beads are also competent binding partners for whole cells, as well as immobilized glycans 14.
To demonstrate the minimal structural requirements for aggregation, sulfated disaccharide 1 was attached to a gold nanoparticle via a thiol linker (15). Particles coated with α-linked fucose residues rapidly aggregated in the presence of Ca2+. Minor structural modifications to the sugar prevented aggregation, and although the β-linked sugar was capable of forming aggregates, the association was weaker than the α-anomer. Sponge cell CCI has also been studied using bovine serum albumin (BSA) conjugates of the disaccharide 1 (16). A solution of 1●BSA aggregated rapidly in the presence of Ca2+ but not Mg2+, which is consistent with previous results observed with the intact proteoglycans. 1●BSA was immobilized on a dextran-coated surface for adhesion studies using surface plasmon resonance (SPR). When a solution of 1●BSA was flowed over the chip, formation of glycoconjugate multilayers on the surface was observed. Similar results were obtained from SPR measurements using intact MAF or g200 (17). Analysis of binding isotherms from the adhesion studies with 1-BSA provided an association constant of 105 M-1 for the interaction of the carbohydrate units. The adhesive forces between glycans involved in sponge CCI have been examined using atomic force microscopy (AFM) (14, 17, 18). AFM tips and mica surfaces were modified with intact MAF or the extracted g200 glycan. Pull-off adhesion forces ranging between 100 and 300 pN have been measured for these interactions. Examination of g200 adhesion from different individuals exhibit distinctions between self-self-interactions and self-non-self-interactions, consistent with the allogeneicity of sponge cell adhesion (17).
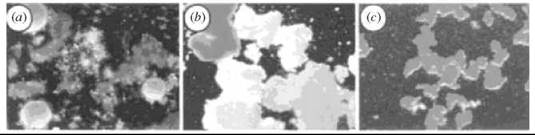
Figure 2. (a) Discrimination between self and non-self, in a suspension of live glyconectin-bearing Microciona prolifera (orange), Halichrondria panicea (white), and Cliona celata (brown) sponge cell at 0°C. The microscopically observed colors are from the whole sponge. (b) Ca2+ dependent species-specific recognition and adhesion of pink, yellow, and white beads coated with glyconectins from each sponge. (c) Control experiment showing sorting of pink, yellow, and white beads, all coated with glyconections from M. prolifera. Magnification x40. Adapted by permission from Macmillan Publishers Ltd. (13).
CCI in Embryo Cell Adhesion
Biological Context
Embryonic development is a process in which cell-cell adhesion is crucial for proper growth of the nascent organism. After fertilization of an egg, the embryo begins to divide into a tightly compacted cell cluster known as the morula. The Lewis x trisaccharide, also referred to as the stage-specific embryonic antigen 1 (SSEA-1), mediates cell adhesion and compaction of the morula during the 8-16 cell developmental stages. Lactofucopentaose III (LNFP-III), a Lewis x containing oligosaccharide, was identified as a carbohydrate inhibitor of fully compacted 8-16 cell embryos. Multivalent inhibitors were constructed by conjugating LNFP-III to a lysyllysine peptide (19). Decompaction of embryo cells was observed only with the multivalent LNFP-III conjugate, and not with free LNFP-III oligosaccharides or other multivalent oligosaccharides. F9 embryonal carcinoma cells resemble cells undergoing early stages of embryonic development, as they also express surface Lewis x antigens at the undifferentiated stage and express less of the antigen upon differentiation. Autoaggregation of these cells was inhibited by LNFP III or EDTA, consistent with a calcium-dependent, homotopic Lewis x interaction (20). Furthermore, solid-supported F9 cells interacted preferentially with Lewis x-containing liposomes. Glycolipids bearing the Lewis x trisaccharide specifically interact with F9 cells, Lewis x-containing liposomes, Lewis x-functionalized columns, and other Lewis x-bearing lipids. Finally, latex beads or gold nanoparticles coated with Lewis x glycolipid aggregate in the presence of calcium ions (21, 22). In each case, the divalent cations Ca2+, Mg2+, or Mn2+ are required for the interaction. The Lewis x CCI was used to engineer the adhesion of non-Lewis x producing cells. Additions of Lewis x glycosphingolipids to a culture of nonaggregating rat basophilic leukemia (RBL) cells resulted in the incorporation of these glycolipids into the plasma membrane and the subsequent clustering of the RBL cells in the presence of calcium (23).
Model Studies
Structural studies using NMR, MS, and X-ray crystallography provided molecular details of the Lewis x CCI. Electrospray ionization mass spectrometry of Lewis x in the presence of calcium ions gave rise to ion peaks consistent with two Lewis x moieties bound to a single calcium atom (24). Additionally, this complex was observed by cold spray ionization, which also detected Lewis x aggregates formed in the absence of calcium (25). NMR spectroscopy of Lewis x in the presence of divalent manganese cations identified tentative metal binding sites based on chemical shift perturbations induced by the paramagnetic ion (26). The binding of monomeric Lewis x to liposomes containing Lewis x has been detected using transfer NOESY experiments, although the strength of the association was exceedingly weak due to the monovalency of one of the binding partners (27). While calcium binding to the trisaccharide alone could not be detected by NMR, linking two molecules of Lewis x by a short tether provided a complex for which nuclear Overhauser enhancements (NOEs) were observed (28). The NMR experiments, along with computational analysis, suggested a model for the complex in which the calcium atom is sandwiched between the two linked trisaccharides with an association constant of 55 M-1. In the solid-state structure of Lewis x, rows of trisaccharides are arranged in a head-to-head arrangement with especially strong interactions between fucose and galactose residues (29, 30). Three intermolecular hydrogen bonds between each Lewis x pair are proposed to mediate their association.
The Lewis x CCI was probed using a variety of quantitative biophysical approaches. Lewis x bearing a thiol terminated lipid tail has been attached to gold surfaces and AFM tips. AFM measurements of the interaction between these tips and surfaces measured an adhesion force of 20 pN (31). The aggregation of Lewis x-coated gold nanoparticles in a calorimeter was found to be highly exothermic (31, 32). SPR measurements of the interaction between Lewis x-coated gold nanoparticles and a self-assembled monolayer on gold functionalized with Lewis x have been carried out, and association constants of 1.9 x 106 M-1 have been reported (33). In an alternative approach, two vesicles containing Lewis x were held in tight contact using micropipette manipulation (34). An adhesion energy of approximately 0.6 kcal/mol was obtained from contact angle measurements of the vesicle interface.
CCI in Melanoma Cell Adhesion
Biological Context
Heterotopic CCIs involving glycolipids were first identified from studies of the adhesion of murine B16 melanoma cells. These cells, which express high levels of the glycosphingolipid GM3 (5), adhered to mouse AA12T-cell lymphomas that express the glycolipid Gg3 (6), but not to the non-Gg3-expressing AA27 variant of the cell line (35). The adheison of AA12 lymphoma cells to a GM3-coated surface was inhibited either by pretreatment of the AA12 cells with an anti-Gg3 monoclonal antibody or by blocking the GM3-coated surface with an anti-GM3 antibody. Conversely, adhesion of B16 melanoma cells to Gg3-coated solid surfaces was correlated to levels of GM3 expression on the B16 cells. Additional evidence for a CCI between GM3 and Gg3 was provided by the adsorption of radiolabeled GM3 lysyllysine glycoconjugates onto a Gg3-coated C-18 column, and the adsorption of GM3 containing liposomes with Gg3 coated plastic surfaces. Besides adhering to lymphoma cells and other multivalent presentations of Gg3, the melanoma cells also bound strongly to plates coated with the glycolipid lactosylceramide (LacCer, 6). Adhesion of liposomes containing GM3 to LacCer-coated solid surfaces was inhibited by an anti-LacCer antibody, identifying a second heterotopic glycolipid partner for GM3.
The adhesion of B16 melanoma cells to endothelial cells can occur both via a CCI involving GM3 as well as through a protein-protein interaction involving integrin receptors. The contribution of CCI is greater during the initial stages of adhesion, whereas integrin-mediated adhesion dominates later. CCI-mediated adhesion is stronger when melanoma cells are passed over immobilized endothelial cells in a dynamic binding assay using a flow chamber (36). Adhesion of the rolling cells can be inhibited by adding LacCer or GM3 containing liposomes to the buffer.
The GM3 in B16 cells is localized within glycolipid-enriched microdomains in the lipid membrane (37). The microdomains, often referred to as lipid rafts, also contain a variety of signaling proteins and enzymes. Binding of B16 cells to immobilized Gg3 activates the signaling proteins Ras and Rho, suggesting that CCI does not simply mediate cell adhesion but may also be involved in signal transduction. The structure and function of the glycolipid signaling domains in B16 cells is perturbed by the addition of an unnatural, synthetically prepared glycosphingolipid (38). The synthetic glycolipid reduced GM3 clustering on the cell surface and diminished the activity of the kinase c-Src, identifying another link between CCI and signal transduction. The specific mechanism by which binding to GM3 activates signal transduction is not currently known.
Several studies suggest that interruption of the LacCer-GM3-mediated adhesion prevents metastasis, raising the possibility of targeting this CCI for cancer therapy. The metastatic potential of several variants of the B16 cell line is correlated with their LacCer binding ability (39). Injection of LacCer containing liposomes to mice that have aggressive melanoma inhibited the development of metastatic lung tumors in the animals (39). Administration of multivalent glycoconjugates such as glycosylated peptides or glycosylated gold nanoparticles also inhibits metastasis in vivo (40, 41). Inhibition of GM3 biosynthesis has also been shown to reduce metastatic melanoma migration in an animal model (42). Further studies with these glycoconjugates are needed to clarify their mechanism of action in vivo.
Model Studies
Langmuir monolayers have been used to model the interactions between GM3 and LacCer or Gg3. Micelles of a lactosyl lipid injected under a GM3 containing monolayer induces a change in the surface pressure (∆π) at the air-water interface in a concentration-dependent manner (43). These increases in ∆π are a consequence of glycolipid insertion into the membrane, but glycoconjugates that do not insert also increase An upon binding (44). Minor changes to the structure of the carbohydrate led to a decrease in the strength of the interaction. The role of the divalent cation in this interaction is unclear, as it is required in some conditions but not others (43, 45, 46). In a separate study, the behavior of various polystyrene glycoconjugates with a GM3 monolayer was examined (47). Pressure-area compression isotherms of GM3 monolayers exhibited expanded areas when they were generated over a solution of the Gg3 glycopolymer. The amount of Gg3 glycoconjugate adsorbed on the GM3 monolayer was quantified using a quartz crystal microbalance by transferring the glycoconjugate-bound monolayer to a quartz substrate (46, 47). An SPR study of this same system provided an association constant for Gg3-glycopolymer binding to a GM3 monolayer of 2.5 x 10-6M-1, (48, 49).
CCI in Myelin Compaction
Biological Context
The myelin sheath is a multilayered membrane that wraps around axons in the nervous system and facilitates the transport of action potentials by preventing dissipation of a signal as it travels along the axon. The lipid bilayers of the myelin sheath are extensions of the membranes of oligodendrocytes in the central nervous system and Schwann cells in the peripheral nervous system. Unlike many other cell membranes, myelin is highly enriched in glycolipids, primarily GalCer (2) and CBS (3), that are preferentially embedded in the outer bilayer (50, 51). In addition, the multilayers are tightly compacted, a process dependent on divalent cations (52). A CCI between GalCer and CBS is believed to play an important role in maintaining the structural integrity of the myelin sheath.
The cellular function of this CCI was studied by examining the interaction of GalCer/CBS containing liposomes with cultured oligodendrocytes (53). Anti-GalCer antibodies bind to glycolipid containing membranes on oligodendrocytes and this binding decreases when the cells are pre-incubated with the glycolipid liposomes. Antibody binding to myelin binding protein (MBP) was similarly inhibited by the liposomes, indicating a colocalization of the glycolipids and MBP in the oligodendrocyte membrane. Liposome incubation also resulted in loss of microtubule fine structures, suggesting that actin filaments are destroyed upon CCI. Similar results were obtained in subsequent studies using galactose (Gal) and sulfated galactose (sGal) conjugates of BSA (54). BSA conjugates containing both Gal and sGal elicited a stronger response than albumin modified with a single carbohydrate.
Model Studies
The interaction between these two glycolipids has also been examined in various chemical model systems. A cerebroside sulfate (CBS) coated surface is adherent to liposomes containing GalCer but not to lactosyl or glucosyl ceramide (55) The aggregation of glycolipid containing liposomes in the presence of various divalent cations was examined (56). The largest amount of aggregation was observed between liposomes containing GalCer and CBS, and the magnitude of the interaction was inversely correlated with the ionic radius of the cations. The presence of longer or a-hydroxylated N-acyl chains on the glycolipids also increased the association between GalCer and CBS liposomes.
Infrared spectroscopy of this interaction revealed that the presence of calcium changed the conformation of the sulfate group in CBS, thereby disrupting several intramolecular hydrogen bonds (57, 58). As membranes in myelin contain both GalCer and CBS, this CCI can occur in a cis- or a trans-fashion. Studies with fluorescent and spin-label probes, as well as anti-glycolipid antibodies, all indicated a preferential trans-interaction between the glycolipids (57). Interestingly, when the GalCer●CBS interaction was probed by IR in lipid dispersions, no cation requirement was observed, in contrast to the results of the liposome aggregation studies. Ternary complexes of GalCer, CBS, and calcium were detected by electrospray mass spectrometry (59, 60). Increased complexation was observed when calcium was replaced with zinc, suggesting a role for the elevated zinc concentrations observed in some types of myelin (60, 61).
References
1. Scott JE. Supramolecular organization of extracellular matrix glycosaminoglycans, in vitro and in the tissues. FASEB J. 1992; 6:2639-2645.
2. Song Y, Withers DA, Hakomori S. Globoside-dependent adhesion of human embryonal carcinoma cells, based on carbohydrate-carbohydrate interaction, initiates signal transduction and induces enhanced activity of transcription factors API and CREB. J. Biol. Chem. 1998; 273:2517-2525.
3. Yu S, Kojima N Hakomori S-I, Kudo S, Inoue S, Inoue, Y. Binding of rainbow trout sperm to egg is mediated by strong carbohydrate-to-carbohydrate interaction between (KDN)GM3 (deaminated neuraminyl ganglioside) and Gg3-like epitope. Proc. Natl. Acad. Sci. U.S.A. 2002; 99:2854-2859.
4. Hakomori S. Carbohydrate-to-carbohydrate interaction, through glycosynapse, as a basis of cell recognition and membrane organization. Glycoconj. J. 2004; 21:125-137.
5. Kawakami Y, Kawakami K, Steelant WFA, Ono M, Baek RC, Handa K, Withers DA, Hakomori S. Tetraspanin CD9 is a “Proteolipid,” and its interaction with alpha 3 integrin in microdomain is promoted by GM3 ganglioside, leading to inhibition of laminin-5-dependent cell motility. J. Biol. Chem. 2002; 277:34349-34358.
6. Wang X, Sun P, Al-Qamari A, Tai T, Kawashima I, Paller AS. Carbohydrate-carbohydrate binding of ganglioside to integrin α5 modulates α5β1 function. J. Biol. Chem. 2001; 276:8436-8444.
7. Wang X-Q, Sun P, O’Gorman M, Tai T, Paller AS. Epidermal growth factor receptor glycosylation is required for ganglioside GM3 binding and GM3-mediated suppression of activation. Glycobiology 2001; 11:515-522.
8. Spillmann D, Burger MM. Carbohydrate-carbohydrate interactions in adhesion. J. Cell. Biochem. 1996; 61:562-568.
9. Misevic GN, Burger MM. The species-specific cell-binding site of the aggregation factor from the sponge Microciona prolifera is a highly repetitive novel glycan containing glucuronic acid, fucose, and mannose. J. Biol. Chem. 1990; 265:20577-20584.
10. Misevic GN, Burger MM. Reconstitution of high cell binding affinity of a marine sponge aggregation factor by cross-linking of small low affinity fragments into a large polyvalent polymer. J. Biol. Chem. 1986; 261:2853-2859.
11. Spillmann D, Hard K, Thomas-Oates J, Vliegenthart JFG, Misevic G, Burger MM, Finne J. Characterization of a novel pyruvylated carbohydrate unit implicated in the cell aggregation of the marine sponge Microciona prolifera. J. Biol. Chem. 1993; 268:13378-13387.
12. Misevic GN, Guerardel Y, Sumanovski LT, Slomianny M-C, Demarty M, Ripoll C, Karamanos Y, Maes E, Popescu O, Strecker G. Molecular recognition between glyconectins as an adhesion self-assembly pathway to multicellularity. J. Biol. Chem. 2004; 279:15579-15590.
13. Popescu O, Misevic GN. Self-recognition by proteoglycans. Nature (London) 1997; 386:231-232.
14. Bucior I, Scheuring S, Engel A, Burger MM. Carbohydrate-carbohydrate interaction provides adhesion force and specificity for cellular recognition. J. of Cell Biol. 2004; 165:529-537.
15. de Souza AC, Halkes KM, Meeldijk JD, Verkleij AJ, Vliegenthart JFG, Kamerling JP. Gold glyconanoparticles as probes to explore the carbohydrate-mediated self-recognition of marine sponge cells. ChemBioChem 2005; 6:828-831.
16. Haseley SR, Vermeer HJ, Kamerling JP, Vliegenthart JFG. Carbohydrate self-recognition mediates marine sponge cellular adhesion. Proc. Natl. Acad. Sci. U.S.A. 2001; 98:9419-9424.
17. Garcia-Manyes S, Bucior I, Ros R, Anselmetti D, Sanz F, Burger MM, Fernandez-Busquets X. Proteoglycan mechanics studied by single-molecule force spectroscopy of allotypic cell adhesion glycans. J. Biol. Chem. 2006; 281:5992-5999.
18. Dammer U, Popescu O, Wagner P, Anselmetti D, Giintherodt H-J, Misevic GN. Binding strength between cell adhesion proteoglycans measured by atomic force microscopy. Science 1995; 267:1173-1175.
19. Fenderson BA, Zehavi U, Hakomori S. A multivalent lacto-n-fucopentaose Ill-lysyllysine conjugate decompacts preimplantation mouse embryos, while the free oligosaccharide is ineffective. J. Exp. Med. 1984; 160:1591-1596.
20. Eggens I, Fenderson B, Toyokuni T, Dean B, Stroud M, Hakomori S. Specific interaction between Le-x and Le-x determinants. A possible basis for cell recognition in preimplantation embryos and in embryonal carcinoma cells. J. Biol. Chem. 1989; 264:9476-9484.
21. Kojima N, Fenderson B, Stroud M, Goldberg R, Habermann R, Toyokuni T, Hakomori S. Further studies on cell adhesion based on Lex-Lex interaction, with new approaches: embryo- glycan aggregation of F9 teratocarcinoma cells, and adhesion of various tumour cells based on Lex expression. Glycoconj. J. 1994; 11:238-248.
22. de la Fuente JM, Barrientos AG, Rojas TC, Rojo J, Canada J, Fernandex A, Penades S. Gold glyconanoparticles as water-soluble polyvalent models to study carbohydrate interactions. Angew. Chem. Int. Ed. Engl. 2001; 40:2258-2261.
23. Boubelik M, Floryk D, Bohata J, Draberova L, Macak J, Smid F, Draber, P. Lexglycosphingolipids-mediated cell aggregation. Glycobiology 1998; 8:139-146.
24. Siuzdak G, Ichikawa Y, Caulfield TJ, Munoz B, Wong CH, Nicolaou KC. Evidence of Ca2+-dependent carbohydrate association through ion spray mass spectrometry. J. Am. Chem. Soc. 1993; 115:2877-2881.
25. Nishimura S-I, Nagahori N, Takaya K, Tachibana Y, Miura N, Monde K. Direct observation of sugar-protein, sugar-sugar, and sugar-water complexes by cold-spray ionization time-of-flight mass spectrometry. Angew. Chem. Int. Ed. Engl. 2005; 44:571-575.
26. Henry B, Desvaux H, Pristchepa M, Berthault P, Zhang YM, Mallet JM, Esnault J, Sinay P. NMR study of a Lewis pentasaccharide derivative: Solution structure and interaction with cations. Carbohydr. Res. 1999; 315:48-62.
27. Geyer A, Gege C, Schmidt RR. Carbohydrate-carbohydrate recognition between Lewisx glycoconjugates. Angew. Chem. Int. Ed. Engl. 1999; 38:1466-1468.
28. Geyer A, Gege C, Schmidt RR. Calcium-dependent carbohydrate-carbohydrate recognition between Lewis x blood group antigens. Angew. Chem. Int. Ed. Engl. 2000; 39:3245-3249.
29. Perez S, Mouhous-Rious N, Nifant’ev NE, Tsvetkov YE, Bachet B, Imberty A. Crystal and molecular structure of a histo-blood group antigen involved in cell adhesion: the Lewis x trisaccharide. Glycobiology 1996; 6:537-542.
30. Yvelin F, Zhang Y-M, Mallet J-M, Robert F, Jeannin Y, Sinay P. Crystal structure of the Lewis x trisaccharide. Carbohydr. Lett. 1996; 1:475-482.
31. Tromas C, Rojo J, de la Fuente JM, Barrientos AG, Garcia R, Penades, S. Adhesion forces between Lewis x determinant antigens as measured by atomic force microscopy. Angew. Chem. Int. Ed. Engl. 2001; 40:3052-3055.
32. De la Fuente JM, Eaton P, Barrientos AG, Menendez M, Penades S. Thermodynamic evidence for Ca2+-mediated self-aggregation of Lewis x gold glyconanoparticles. A model for cell adhesion via carbohydrate-carbohydrate interaction. J. Am. Chem. Soc. 2005; 127:6192-6197.
33. Hernaiz MJ, de la Fuente JM, Barrientos AG, Penades S. A model system mimicking glycosphingolipid clusters to quantify carbohydrate self-interactions by surface plasmon resonance. Angew. Chem. Int Ed. Engl. 2002; 41:1554-1557.
34. Gourier C, Pincet F, Perez E, Zhang Y, Zhu Z, Mallet J-M, Sinay P. The natural Lewis x-bearing lipids promote membrane adhesion: influence of ceramide on carbohydrate-carbohydrate recognition. Angew. Chem. Int. Ed. Engl. 2005; 44:1683-1687.
35. Kojima N, Hakomori S. Specific interaction between gangliotriao-sylceramide (Gg3) and sialosyllactosylceramide (GM3) as a basis for specific cellular recognition between lymphoma and melanoma cells. J. Biol. Chem. 1989; 264:20159-20162.
36. Kojima N, Shiota M, Sadahira Y, Handa K, Hakomori S. Cell adhesion in a dynamic flow system as compared to static system. J. Biol. Chem. 1992; 267:17264-17270.
37. Iwabuchi K, Yamamura S, Prinetti A, Handa K, Hakomori S. GM3-enriched microdomain involved in cell adhesion and signal transduction through carbohydrate-carbohydrate interaction in mouse melanoma B16 cells. J. Biol. Chem. 1998; 273:9130-9138.
38. Zhang Y, Iwabuchi K, Nunomora S, Hakomori S. Effect of synthetic sialyl 2-1 sphingosine and other glycosylsphingosines on the structure and function of the glycosphingolipid signaling domain (gsd) in mouse melanoma B16 cells. Biochemistry 2000; 39:2459-2468.
39. Otsuji E, Park YS, Tashiro K, Kojima N, Toyokuni T, Hakomori S. Inhibition of B16 melanoma metastasis by administration of GM3-Gg3Cer or GM3-LacCer interaction. Int. J. Oncol. 1995; 6:319-327.
40. Dean B, Ogushi H, Cai S, Otsuji E, Tashiro K, Hakomori S-I, Toyokuni T. Synthesis of multivalent β-lactosyl clusters as potential tumor metastasis inhibitors. Carbohydr. Res. 1993; 245:175- 192.
41. Rojo J, Diaz V, de la Fuente JM, Segura I, Barrientos AG, Riese HH, Bernad A, Penades S. Gold glyconanoparticles as new tools in antiadhesive therapy. ChemBioChem 2004; 5:291-297.
42. Deng W, Li R, Guerrera M, Liu Y, Ladisch S. Transfection of glucosylceramide synthase antisense inhibits mouse melanoma formation. Glycobiology 2002; 12:145-152.
43. Santacroce PV, Basu A. Probing specificity in carbohydrate-carbohydrate interactions with micelles and langmuir monolayers. Angew. Chem. Int. Ed. Engl. 2003; 42:95-98.
44. Santacroce PV, Basu A. Glycodendrimers as tools for studying carbohydrate-carbohydrate interactions. Polymer Preprints 2005; 46, 1114.
45. Santacroce PV, Basu A. Studies of the carbohydrate-carbohydrate interaction between lactose and GM3 using langmuir monolayers and glycolipid micelles. Glycoconj. J. 2004; 21:89-95.
46. Matsuura K, Kitakouji H, Oda R, Morimoto Y, Asano H, Ishida H, Kiso M, Kitajima K, Kobayashi K. Selective expansion of the GM3 glycolipid monolayer induced by carbohydrate-carbohydrate interaction with Gg3 trisaccharide-bearing glycoconjugate polystyrene at the air-water interface. Langmuir 2002; 18:6940-6945.
47. Matsuura K, Kitakouji H, Tsuchida A, Sawada N, Ishida H, Kiso M, Kobayashi K. Carbohydrate-carbohydrate interaction between glycolipids and glycoconjugate polystyrenes at the air-water interface. Chem. Lett. 1998; 12:1293-1294.
48. Matsuura K, Oda R, Kitakouji H, Kiso M, Kitajima K, Kobayashi, K. Surface plasmon resonance study of carbohydrate-carbohydrate interaction between various gangliosides and Gg3-carrying polystyrene. Biomacromolecules 2004; 5:937-941.
49. Matsuura K, Kitakouji H, Sawada N, Ishida H, Kiso M, Kitajima K, Kobayashi, K. A quantitative estimation of carbohydrate-carbohydrate interaction using clustered oligosaccharides of glycolipid monolayers and of artificial glycoconjugate polymers by surface plasmon resonance. J. Am. Chem. Soc. 2000; 122:7406-7407.
50. Inouye H, Kirschner DA. Membrane interactions in nerve myelin: II. Determination of surface charge from biochemical data. Biophys. J. 1988; 53:247-260.
51. Morell P (1984). Myelin. 2nd edition. 1984. Plenum Press, New York.
52. Melchior V, Hollingshead CJ, Caspar DLD. Divalent cations cooperatively stabilize close membrane contacts in myelin. Biochim. Biophys. Acta 1979; 554:204-226.
53. Boggs JM, Wang H. Effect of liposomes containing cerebroside and cerebroside sulfate on cytoskeleton of cultured oligodendrocytes. J. Neurosci. Res. 2001; 66:242-253.
54. Boggs JM, Wang H. Co-clustering of galactosylceramide and membrane proteins in oligodendrocyte membranes on interaction with polyvalent carbohydrate and prevention by an intact cytoskeleton. J. Neurosci. Res. 2004; 76:342-355.
55. Hakomori S. Carbohydrate-carbohydrate interaction as an initial step in cell recognition. Pure App. Chem. 1991; 63:473-482.
56. Stewart RJ, Boggs JM. A carbohydrate-carbohydrate interaction between galactosylceramide-containing liposomes and cerebroside sulfate-containing liposomes: Dependence on the glycolipid ceramide composition. Biochemistry 1993; 32:10666-10674.
57. Boggs JM, Menikh A, Rangaraj G. Trans interactions between galactosylceramide and cerebroside sulfate across apposed bilayers. Biophys. J. 2000; 78:874-885.
58. Menikh A, Nyholm P-G, Boggs JM. Characterization of the interaction of Ca2+ with hydroxy and non-hydroxy fatty acid species of cerebroside sulfate by fourier transform infrared spectroscopy and molecular modeling. Biochemistry 1997; 36:3438-3447.
59. Koshy KM, Boggs JM. Investigation of the calcium-mediated association between the carbohydrate head groups of galactosyl-ceramide and galactosylceramide I3 sulfate by electrospray ionization mass spectrometry. J. Biol. Chem. 1996; 271:3496-3499.
60. Koshy KM, Wang J, Boggs JM. Divalent cation-mediated interaction between cerebroside sulfate and cerebrosides: an investigation of the effect of structural variations of lipids by electrospray ionization mass spectrometry. Biophys. J. 1999; 77:306-318.
61. Inouye H, Kirschner DA. Effects of ZnCl2 on membrane interactions in myelin of normal and shiverer mice. Biochim. Biophys. Acta 1984; 776:197-208.
Further Reading
Rojo J, Morales JC, Penades S. Carbohydrate-carbohydrate interactions in biological and model systems. Top. Curr. Chem. 2002; 218,45-92.
Special Issue on carbohoydrate-carbohydrate interactions. Glyconj. J. 2004; 21(3-4).
Spillmann D, Burger MM. Carbohydrate-carbohydrate interactions. In: Carbohydrates in Chemistry and Biology. Ernst B, Hart GW, Sinay P, eds. 2000. Wiley-VCH, New York.
See Also
Lipid Domains, Chemistry of
Neoglycoproteins, Chemistry of
Sugar-Lectin Interactions in Cell Adhesion
Glycomimetics
Surface Techniques and Surface Chemistry