CHEMICAL BIOLOGY
Hormone Signaling
Ross V. Weatherman, Department of Medicinal Chemistry and Molecular Pharmacology and the Purdue Cancer Center, Purdue University, West Lafayette, Indiana
doi: 10.1002/9780470048672.wecb229
Hormone signaling has always been a field that has required working at the chemistry-biology interface. The development of new chemical tools plays a major role in understanding the molecular mechanisms of hormone signaling and the physiologic outcomes of hormone receptor action. This article will outline the relevance of molecular endocrinology to the field of chemistry and the use of chemical approaches to solving problems in hormone research. The basic biologic outline of a hormone signaling system and the different classes of hormone molecules and their receptors will be discussed. In addition, several research areas in which new chemical tools have been playing key roles will be described in more detail, including the discovery of ligands for orphan hormone receptors, the development of non-natural hormone receptor mimics, and the use of selective hormone receptor modulators to understand the role of specific hormone receptor signaling pathways. Hormone signaling systems that will be discussed include the thyronamines and trace amine receptor, the liver X and farnesol X receptors, small molecules capable of binding to peptide hormone receptors, the melanocortin receptor family, the estrogen receptor, and chemically orthogonal hormone receptor-ligand pairs. Major challenges facing the field as well as some different experimental methods used to study hormone signaling will also be discussed.
At a fundamental level, multicellular life would not be possible if it were not for the ability of different cells to communicate and coordinate using a language based on biomolecular interactions. From the simplest two-component signaling systems found in primitive multicellular colonies to the complicated networks of overlapping signaling cascades found in higher vertebrates, hormone signaling is dependent on the production, diffusion, and recognition of small molecules and peptides. As such, hormone signaling has always been a productive area of study for people working at the chemistry-biology interface. Chemistry has played an important role in elucidating the molecular mechanisms and consequences of hormone signaling and will continue to be a vital tool in tackling the complicated new challenges that face the field. This advanced review will introduce some key features of hormone signaling that are relevant to the chemical biology interface and then detail several examples in which chemical approaches have provided the key solutions to very challenging problems. In addition, this review will focus on current and future challenges in the study of hormone signaling and on possible opportunities for people working at the chemistry-biology interface to assist in overcoming these challenges.
The Basic Biology of Hormone Signaling
A hormone, simply defined, is a chemical messenger that relays signals from one cell to another. With such a broad definition, almost any biomolecule could be a hormone in certain contexts, but this article will focus on classically recognized endocrine and paracrine hormones that typically are produced in specific groups of cells in one organ and target nearby cells (paracrine) or cells or tissues in distant organs (endocrine). Even with a focus just on these classes of hormones, the biology of hormone signaling is too vast to be covered by writing a large textbook let alone a small review article. As a result, this section will concentrate on issues relevant to the point of view of the chemist: why a chemist should care about hormone signaling, some basic features of hormone signaling systems, and the archetypal classes of hormones and hormone receptors.
Why chemists should care about hormone signaling
In a schematic of a typical hormone signaling pathway, one often sees a jumble of arrows pointing to various geometric shapes labeled with strange names and acronyms. These sorts of signal transduction schemes can be daunting to decipher and often discourage people with more chemically oriented backgrounds from pursuing research in the field. This reaction is unfortunate because a “hormone-centric” view of hormone signaling would reveal a structurally diverse set of hormones, many of them small molecules, that can act as molecular switches for large cascades of protein-protein interactions simply by changing the conformation of their hormone receptors. As will be discussed in greater detail later, subtle changes in hormone structure can affect hormone signaling and have dramatic physiologic consequences. The pathways that lead to those physiologic changes can be complex, but they all arise from the interaction of molecules. Understanding, mimicking, and controlling those molecular interactions are clearly areas where chemistry continues to play a major role.
Beyond the potential scientific appeal of hormone signaling to the chemist, obvious practical applications of chemistry in the field exist. Many significant leaps in knowledge in endocrinology occurred after the development of new chemical tools. Advances in chromatography and analytical chemistry were fundamental for the discovery and characterization of many hormones, and the development of radiolabeling techniques and molecular biology led to the isolation and characterization of many hormone receptors (1-5). As will be discussed, the development of new compounds with selective modulation of specific hormone signaling pathways has greatly increased our knowledge of the molecular underpinnings of hormone action (10-12). Hormones also play a key role in the development and treatment of a large number of diseases ranging from breast and prostate cancer to diabetes and obesity (13, 14). A significant percentage of the top-selling drugs in the United States target hormone signaling, including drugs used to treat inflammation, diabetes, hypertension, hypothyroidism, and other hormone-related indications (13-15).
Basic features of hormone signaling
At its most basic level, hormone signaling must involve a hormone and some sort of target. In most cases the target is a receptor protein, although some hormones have been proposed to function also by non-receptor-mediated mechanisms such as altering the local redox environment of a particular cell (16, 17). Basic hormone signaling follows a simple signaling loop (see Fig. 1). Every component of a hormone signaling loop can be affected through the use of chemical tools. In an endocrine or paracrine signaling system, two different cells exist: one making the hormone and one possessing the receptor and carrying out the biologic response. In the hormone-producing cell, typically some sort of stimulus exists to initiate production of the hormone, usually some sort of change in the concentration of a marker sensed by the cell or perhaps another hormone. The hormone is then produced either by biosynthetic enzymes or by release from storage vesicles inside the cell. The hormone then must be secreted out of the cell and transported to the target cell. This secretion and transport either can be a passive process or can involve other proteins. After reaching the target cell, the hormone can bind to the receptor, if it is extracellular, or it can be taken into the cell by either passive or active transport and bind to intracellular receptors. Once bound to the receptor, the signal must be transduced into a biologic response. Finally, if the desired biologic response has been accomplished, some sort of feedback signal is commonly sent back to the hormone-producing cell to halt hormone production.
As mentioned, it is possible to modulate each of these processes using chemical tools and to affect the whole signaling loop. Sometimes this modulation can be intentional, such as the use of aromatase inhibitors to halt the production of estrogens and block estrogen signaling (18), or it can be unintentional, as is often the case with hormone receptor antagonists also blocking negative feedback inhibition and causing overproduction of the natural hormone (19). As such, it is important for any chemist who wishes to apply chemical tools to the study of hormone signaling in whole organism models, as in the case of new therapeutics, to consider hormone signaling as a system with many different components.

Figure 1. The basic features of a hormone signaling pathways. Each feature can be potentially modulated using chemical tools.
Classes of hormones and hormone receptors
Generally, hormones can be organized into three families: the peptide hormones, the steroid hormones, and a very loosely organized class of hormones derived from the modification of amino acids and lipids (see Fig. 2). The structure of the hormone dictates in many ways the nature of the hormone receptor. The peptide hormones can vary widely in length and amino acid composition, but they are generally too hydrophilic to cross the plasma membrane. As a result, most hormone receptors for peptide hormones are membrane receptors such as G-protein coupled receptors (GPCRs), receptor tyrosine kinases, and ion channels. As will be discussed, a key problem in the area of peptide hormone research is how to improve the physical properties of therapeutics by targeting peptide hormone receptors using peptidomimetics. The steroid hormone family is sufficiently hydrophobic to cross the membrane receptors, so its receptors tend to be intracellular nuclear receptors, although extracellular receptors for steroid hormones have been proposed (20). The third family of hormones covers all remaining small molecules and, as such, targets several different extracellular receptors or intracellular receptors depending on the particular hormone. One class of hormones synthesized from amino acids includes biogenic amines such as dopamine, serotonin, histamine, and epinephrine. Although most of these biogenic amines are more closely identified as neurotransmitters, the biogenic amines are present in physiologically relevant concentrations in circulation and have receptors located in several tissues in the cardiovascular, digestive, and immune systems (21-24). Molecules well known to chemical biologists for other roles, such as adenosine and ATP, also are known to have hormone signaling functions (25). Also several hormone receptors exist for which no hormone has ever been discovered. These so-called “orphan receptors” are numerous, and discovering ligands to match these orphan receptors is one important area of hormone research that requires chemical tools.

Figure 2. Examples of the different basic structural classes of hormones.
Chemical Applications in the Study of Hormone Signaling
As stated, major leaps forward in the study of hormone signaling have often coincided with the development of new chemical tools. As such, an exhaustive history of these tools is far beyond the scope of this article. Instead, the article will focus on a few studies in which newly developed chemical tools have had an important impact on the understanding of different hormone signaling systems. The types of approaches used in these studies can be applied to several different types of hormone signaling systems and hopefully will serve as examples of the potential of studying hormone signaling at the chemistry-biology interface.
Discovering new ligands for orphan hormone receptors
One of the consequences of the Human Genome Project has been the identification of genes that encode proteins with no known function. Some of these proteins share sequence homology with known hormone receptors, which strongly suggests that these new receptors should have ligand partners. These “orphan” receptors exist in every major family subtype but they seem to be especially common in the GPCR and nuclear receptor families (26, 27). Although it is likely that some of these hormone receptors will not have a ligand, undoubtedly other orphan receptors exist that do have a hormone that has not yet been discovered. Finding methods to match ligands to orphan receptors would have a huge impact in discovering new drug targets and remains a major challenge in the field of molecular endocrinology. Several approaches are currently being used to attempt to accomplish this task. These methods include in vitro screening of large libraries of compounds, transgenic mouse models, structure-based screening methods, and bioinformatics; they have been applied most often to orphan members of the GPCR and nuclear receptor families (27, 28). Although some successes have been reported, it is important to remember that any potential ligand found during in vitro studies must also be present at sufficient concentrations in vivo for a sufficiently long enough period of time to qualify as an endogenous ligand.
Another strategy to discovering ligand matches for orphan receptors is to start with metabolites of known hormones, assuming that only a limited number of hormone structures exists that an organism can synthesize. This approach was used with great success in finding hormone ligands for the orphan nuclear receptors liver X receptor (LXR) and farnesoid X receptor (FXR). Based on expression patterns of the receptor in different tissues, Manglesdorf et al. hypothesized that LXR and FXR played some sort of role in cholesterol and bile acid metabolism. Through a combination of tissue extracts and screening and organic synthesis, they discovered that LXR could be regulated by sterols such as 24(S)-hydroxycholesterol and that FXR could be modulated by bile acids such as chenodeoxycholic acid at physiologically relevant concentrations (29, 30). The matching of ligands with these two receptors has had a huge impact in the field of lipid metabolism and has led to the development of potentially promising therapeutic candidates.
Another example of using hormone metabolites to discover new hormone signaling pathways is the case of a metabolite of thyroid hormone. Scanlan et al. noted the similarities between 3,3',5-triiodothyronine (T3), the highest affinity endogenous ligand for the thyroid hormone receptor, and various biogenic phenethylamines such as dopamine if T3 were to be enzymatically decarboxylated to a thyronamine (see Fig. 3). Several different thyronamines were synthesized with different degrees of iodination and screened against GPCRs thought to bind to biogenic amines. One of these compounds, 3-iodothyronamine, was bound with high affinity to an isoform of the trace amine receptor (TAAR1), an orphan GPCR with no previously identified endogenous ligand (31). Additional biologic characterization of this interaction showed that the ligand was found endogenously in rat and guinea pig brain and that when the compound was administered to mice it caused rapid slowing of heartbeat and an almost 8-degree drop in body temperature. The effects were reversible over time with no deleterious long-term effects on the mice. Interestingly, the effects of 3-iodothyronamine on the organism were opposite to those of T3. The thyroid hormone receptor is a member of the nuclear receptor superfamily and exerts most of its effects at the transcriptional level where it causes an increase in body temperature and heart rate. It seems like T3 and its iodothyronamine metabolite act in concert to maintain a balance in homeostasis as it relates to body temperature and heart rate—two of the most fundamental processes of an organism. Higher potency analogs have also been synthesized to explore this whole new area of hormone signaling research that was made possible by new chemical tools (32).
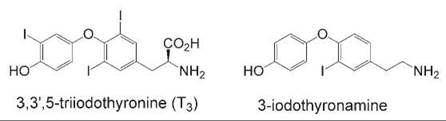
Figure 3. Comparison of the highest potency thyroid hormone, T3, and its thyronamine metabolite, a potent ligand of a trace amine receptor subtype.
Non-natural hormone mimetics
In contrast to the case of orphan receptors without an endogenous ligand, many hormone receptors have well-characterized endogenous ligands. Even with well-characterized hormones, however, a strong need remains for synthetic analogs for these hormones that have different structures than their endogenous counterparts. Many reasons exist for needing synthetic analogs, including the possibility of designing a hormone antagonist that can be used as a tool to block hormone signaling. Another reason that is especially relevant to peptide hormones is the need to change the physical properties of the hormone. Although several peptide hormones are used therapeutically, such as insulin and oxytocin, peptides generally are not orally bioavailable and usually are administered parenterally (33). The ideal hormone mimic would be orally available and would show increased half-life in the circulation.
Most successes in designing non-natural hormone mimics have come from nonpeptide hormone families. Nonsteroidal ligands exist for almost all steroid hormone receptors, and many are used therapeutically (34, 35). The same is true for hormones based on amino acids and lipids. The one hormone class where mimicry has been difficult to achieve is the peptide hormone class. Several strategies have been employed using peptide scaffolds to alter the physical properties of peptide hormones, including truncation, cyclization, and substitution with non-natural amino acids (36). These sorts of strategies have been greatly aided by the development of rapid peptide synthesis and screening techniques such as phage display (37). Many attempts have been made to create nonpeptide mimics of peptide hormones. It is a well-known problem that protein-protein interfaces are difficult interactions to mimic or block with a small molecule (38), but some notable successes exist in the field of hormone signaling (39, 40). A small molecule capable of mimicking the biologic activity of granulocyte colony stimulating factor (G-CSF) can induce the oligomerization of the hormone receptor in a manner similar to the endogenous peptide hormone (41). Also, small-molecule mimics have been discovered for the insulin receptor (42), fibroblast growth factor (43), and interleukin 2 (44). Although these mimics all seem to possess the functional equivalence of the endogenous hormones, it is still possible that these small molecules achieve their biologic effects by mechanisms other than direct binding to the hormone-binding site on the receptor.
Synthetic hormone signaling systems
In some cases, a particular application may call for some sort of hormone-regulated control that is not present, as in the case of selective transcriptional modulation of a specific transgene or signal transduction event. Several research groups have developed approaches to this problem by engineering a synthetic hormone signaling system that is orthogonal to all endogenous hormone pathways. If an effector is also placed under the regulation of this orthogonal receptor, then the response can be modulated by the addition of the matching ligand. The approaches have varied from using hormone receptors from other species to engineering receptors and ligands via a “bump-hole” approach. These approaches have been used to generate orthogonal versions of estrogen and the thyroid hormone receptor and can be used to explore the functions of a specific receptor (45-48). These synthetic hormone signaling systems have also been used to regulate transcription of specific transgenes as well as to control other hormone receptor families such as GPCRs, as was the case with the adenosine A3 receptor (49).
Selective hormone receptor modulation
Sometimes, simple mimicry of the hormone is not enough. One major reason that hormone signaling has been such a fruitful area of research is that many of these hormones have multiple effects. Endocrine hormones are systemically circulated, so it is not surprising that they can modulate receptors in many different tissues. Although this finding means that a hormone can have many different functions worthy of study, it also means that looking at the role or mechanism of one specific response is more difficult because of possible interference from other signaling events elicited by that hormone. This interference can have significant therapeutic ramifications if the other signaling events cause deleterious side effects. Several examples exist in endocrinology where selective modulation of hormone signaling is desired and where one of the most effective ways to achieve selective modulation is to use a selective ligand. The term “selective” can have different meanings when it comes to hormone signaling. This section will look at two different examples of selectivity: receptor isoform selectivity and “response” selectivity.
One key feature of many hormone signaling systems is that often multiple receptors can bind the same hormone. The same hormone can sometimes bind totally different receptors, as is the case with estradiol binding to the estrogen receptor, a member of the nuclear receptor superfamily, and to GPR30, a GPCR (50). Typically, however, the same hormone binds to variants of a single hormone receptor, either isoforms created by alternative splicing or subtypes actually encoded by different genes. Even though these variants may bind to the same hormone and have similar structural features, they can have significantly different functions. They may be expressed in different tissues or expressed at different times in the development of an organism and thus can regulate significantly different signal transduction pathways from other variants of the same receptor. To determine the function of individual variants in a biologic system, it is necessary to uniquely activate the variant of interest. Although genetic approaches that use knockout animal models have been extremely valuable, the ultimate tool for uncovering the role of a specific hormone receptor variant is a selective ligand. In the field of molecular endocrinology, the development of a selective ligand is almost always greeted with much enthusiasm because of the possible promise that it might finally uncover what that particular receptor variant does.
Numerous examples exist where variant-selective ligands have been used to dissect complex hormone signaling pathways, but one of the best examples of the use of selective ligands to uncover the function of very disparate receptor variants is the case of the melanocortin receptor. The melanocortin receptor family is a group of five receptor subtypes that belong to the GPCR superfamily and bind to several similar peptide hormones, α, β, and γ-melanocyte stimulating hormones (MSHs) (51). The receptors are expressed at different amounts in a wide variety of tissues and seem to have roles in obesity, inflammation, and cardiovascular function, as well as the more expected role of controlling skin pigmentation. To better understand what specific receptor subtypes are doing, as well as to explore the potential of using melanocortin receptors as drug targets, several attempts have been made to design and synthesize selective receptor modulators. The different receptor subtypes have different binding preferences for the various hormones, which suggests that it might be possible to differentiate the receptor subtypes on the basis of ligand-binding affinity. Several selective ligands have been reported, although few of them are specific for just one subtype. Enough selectivity has been achieved, however, to start to understand the roles that some receptor subtypes are playing in the melanocortin hormone signaling system.
Because the melanocortin receptor subtypes are peptide hormone receptors, great effort has gone into using various peptide synthesis and screening methods to uncover selective ligands (52). This work has led to the development of several compounds with different patterns of selectivity toward the different receptor subtypes. One of the first reported selective compounds, a peptide termed MTII, showed selectivity for both the MCR3 and the MCR4 subtypes (53); it was an anti-inflammatory agent in a rodent model and could block overeating in an animal obesity model, which suggests that the two subtypes may play a role in both energy homeostasis and inflammation (54, 55). In addition, another peptide, labeled SHU9119, acted as an antagonist at the MC3 R and MC4R subtypes, acted as an agonist at the MC1 R and MC5 R subtypes, and was able to block the anti-inflammatory and anti-obesity effects of MTII. Selective nonpeptide ligands have also been developed for the MC1 R subtype, which prevented inflammation in acute inflammatory mouse models (56), as well as for the MC4 R subtype, which seemed to affect sexual function (see Fig. 4) (57). Although the various compounds are not quite selective enough to precisely determine what each melanocortin receptor subtype is doing, the development of selective chemical tools has greatly increased understanding of this complex hormone signaling network.

Figure 4. Selective nonpeptide modulators of melanocortin receptor subtypes. The MC4 R selective compound seems to affect sexual function, and the MC1 R selective compound possess anti-inflammatory activity.
In some cases, it seems that different responses to a given hormone can come from just one receptor subtype. In such cases, the hormone is binding to the same receptor variant, but the response is different depending on the cell or tissue context. This has been reported for several hormone receptors, but the classic example is the estrogen receptor (ER) (58). Estrogens have a variety of responses in different tissues ranging from proliferation in some tissues to inhibition of proliferation in others. Although several different receptors for estrogen hormones exist, including the orphan GPCR GPR30 and the ER subtype ER beta, several tissue-dependent responses result from binding to a single receptor subtype—ER alpha (59). The challenge is to understand how the receptor can bind identically to the same compound but have totally different signaling responses depending on the cell context. Fortunately, several chemical tools that show patterns of selective modulation, called selective estrogen receptor modulators (SERMs), have been developed and can help dissect this challenging problem.
Some of the most widely used SERMs have already been clinically validated: tamoxifen, the most widely used drug to treat and prevent hormone-responsive breast cancer, and raloxifene, used for the prevention of osteoporosis and being considered as a breast cancer preventive (60). Both compounds act as anti-estrogens in the breast and block estrogen-induced proliferation but act as estrogens in the bone where they prevent osteoporosis. In the uterus, they have different activities: tamoxifen is estrogenic and induces proliferation in the uterus, whereas raloxifene blocks proliferation (61). Other compounds have different patterns of responses in different tissues, and all seem to be able to bind to the estrogen receptor with high affinity. So key questions remain: How can one receptor have so many different responses that are dependent on cell context? How can it be controlled?
The combination of chemical tools and structural biology are beginning to provide the answers to these important questions. When comparing mechanisms to explain estrogenic responses versus anti-estrogenic responses, the structures of the ligand-binding domain of the estrogen receptor bound to estradiol, tamoxifen, or raloxifene were compared (see Fig. 5) (62). The SERMs tamoxifen and raloxifene cause a major perturbation in one alpha helix of the domain. The cleft created by that helix when an estrogen binds is recognized by a coactivating protein that allows further buildup of a complex that activates transcription at a particular promoter. The cleft is obstructed when tamoxifen or raloxifene binds to the ligand domain; this binding leads to an interaction with a corepressor protein that, in turn, promotes the buildup of a complex that represses transcription. This mode of antagonism has become a common feature in drug design for nuclear receptors (10).
Although this structural comparison only reveals possible mechanisms by which estradiol can differ from tamoxifen and raloxifene, it also suggests that a single receptor can have several different biologic responses caused by different downstream effectors that interact with different parts of the receptor. Differential expression of those effectors can then dictate different responses to the same drug in different cells and tissues. Even though the tamoxifen and raloxifene structures are similar, the crystal structures contain only one domain of a three-domain receptor; the other domains could be playing a role in transducing slight changes in conformation of the ligand-binding domain into more significant changes in the overall receptor conformation. It is also known that the estrogen receptor modulates many different pathways, including the activity of different transcription factors (63). Some success has been reported at developing inhibitors to block interactions between ER and downstream effectors (64). Finding more chemical approaches like these to identify ligand-selective effectors as well as developing new chemical tools that can find new downstream estrogen signaling pathways are key areas where chemical biology will play a key role in untangling a complicated and therapeutically important hormone signaling network.

Figure 5. A representation of the estrogen receptor alpha ligand-binding domain bound to estradiol, 4-hydroxytamoxifen, or raloxifene. Key helix 12 is highlighted in white. The hatched area indicates the coactivator-binding cleft formed upon estradiol binding, which is blocked by helix 12 upon 4-hydroxytamoxifen or raloxifene binding.
Chemical Tools and Techniques
For a field as broad as hormone signaling, the key chemical tools and techniques used in the field could include almost anything. The important thing to remember about studying the chemical biology of hormone signaling is that relying on only in vitro data usually will give you only a small part of the story. Ultimately, cell-based and preferably animal-based studies should be conducted to test any tools that might be developed. In general, several techniques are shared by most hormone signaling studies, but each hormone and hormone receptor will have unique assays. Very brief descriptions of these techniques will follow. Most of the assays described are very generalized and not described by single reference sources. The most useful source for many of these assays will be medicinal chemistry papers that describe a hormone receptor modulating compound from synthesis to animal testing, but another good source for protocols are the retailers of commercially available assay kits and can be easily found online.
Receptor-ligand binding assays
In almost all cases hormone signaling involves the binding of receptors and ligands, and the development of any chemical tool will need to include some assay to measure binding affinity. Typically, in vitro binding assays with either radiolabeled or fluorescently labeled ligand and purified receptor are used, although sometimes membrane extracts have to be used for membrane-localized receptors (65-68). Several of these assays have been developed, are available commercially, and are amenable to high-throughput screening. It is important to calculate binding affinity with dose response curves using nonlinear regression analysis whenever possible. Several affordable software packages are available to do this sort of statistical analysis. One caveat to these types of binding assays is that receptor binding affinity in vitro does not always correlate well to binding affinity in cells.
Reporter assays
Most hormone signaling studies will also need some assay to measure the cellular effects of the ligand being developed. These studies usually are performed in cultured cells and can range from transcriptional reporter assays using luciferase reporter plasmids (69) to enzymatic assays for the activation of various kinases and cyclases (70, 71) to cellular sensor assays looking for changes in the concentration of markers such as intracellular calcium (72). In these assays the cells are typically dosed with a compound for a certain period of time in multiwell culture plates; then the cells are lysed, and the reporter is measured using some sort of spectrophotometric technique. Also whole-cell and whole-animal imaging techniques are beginning to be used to perform more complex reporter assays (73).
Mutagenic scanning methodologies
One key question that should be addressed when searching for a new hormone receptor or hormone receptor subtype is as follows: Which amino acids are involved in hormone binding? Homology modeling with similar receptors has been extremely useful in identifying potential ligand binding sites in orphan nuclear receptors, but one of the most useful techniques for peptide hormone receptors is scanning mutagenesis. In this technique, amino acids in a protein can be systematically replaced with other amino acids, typically alanine, and receptor binding can be assessed. The mutagenesis can be performed using several different approaches, but some sort of high-throughput screening of receptor binding or activity must be used in order to achieve thorough coverage (74). Mutageneis has been used extensively to study the interaction between the human growth hormone and its receptor (75, 76). It has also been used with peptide hormones such as insulin and vasopressin (77, 78), as well as nonpeptide hormone receptors such as the vitamin D receptor (79).
Proteomics, genomics, and systems biology
Proteomic and genomic technology are other tools derived by chemists that are having a dramatic effect on the study of hormone signaling. Many questions regarding downstream effectors of a specific hormone receptor in a specific cell type can be answered using proteomic techniques to identify uniquely expressed proteins in specific tissues (80). If the hormone receptor modulates transcription, genomics can be very valuable in determining the subsets of genes affected by the receptor in a given tissue (81). In addition, the emerging field of systems biology should make significant contributions to the molecular study of hormone signaling at the organism level (82).
Major Challenges and Future Direction
Hormone signaling was one of the original fields of study that required working at the chemistry-biology interface, and it will continue to need people trained in multidisciplinary work to unravel many big questions facing the field. The topics described above—matching ligands to orphan receptors, finding unnatural mimics of hormones, and improving and understanding selective receptor modulation—will continue to remain major challenges in the field and to require the development of new chemical tools. The other major challenges are the development of real-time techniques for detecting the biological responses to hormones and hormone mimics, as well as the development of compounds and techniques to dissect the roles of multiple hormone signaling systems in a particular physiological response. It is becoming abundantly clear that most hormone receptors crosstalk with other receptors from the same hormone family as well as with other hormone families. For instance, the crosstalk between growth factor receptor signaling and estrogen receptor signaling is believed to play a major role in the development of several kinds of antiestrogen resistance in breast cancer (83). Major health problems facing western populations, such as obesity, are also endocrine disorders and will involve several different hormone receptor systems (84). These tough problems require people trained in the chemical sciences to develop the tools necessary to solve them. If the past is any guide, it is likely that a new chemical tool will be behind every great push forward in our understanding of the molecular mechanisms of hormone signaling.
References
1. Jensen EV, Greene GL, Closs LE, DeSombre ER, Nadji M. Receptors reconsidered: a 20-year perspective. Recent Prog. Horm. Res. 1982; 38:1-40.
2. Jensen EV, DeSombre ER. Estrogen-receptor interaction. Science 1973; 182:126-134.
3. Brown H, Sanger F, Kitai R. The structure of pig and sheep insulins. Biochem. J. 1955; 60:556-565.
4. Bergstrom S. Prostoglandins: members of a new hormonal system. These physiologically very potent compounds of ubiquitous occurrence are formed from essential fatty acids. Science 1967; 157:382-391.
5. Axelrod J. Noradrenaline: fate and control of its biosynthesis. Science 1971; 173:598-606.
6. Tanner JM. Human growth hormone. Nature 1972; 237:433-439.
7. Schally AV, Arimura A, Kastin AJ. Hypothalamic regulatory hormones. Science 1973; 179:341-350.
8. Guillemin R. Peptides in the brain: the new endocrinology of the neuron. Science 1978; 202:390-402.
9. Oppenheimer JH, Schwartz HL, Surks MI, Koerner D, Dillmann WH. Nuclear receptors and the initiation of thyroid hormone action. Recent Prog. Horm. Res. 1976; 32:529-565.
10. Gronemeyer H, Gustafsson JA, Laudet V. Principles for modulation of the nuclear receptor superfamily. Nat. Rev. Drug Discov. 2004; 3:950-964.
11. Katsoyannis PG. Synthesis of insulin. Science 1966; 154:1509-1514.
12. Sutherland EW. Studies on the mechanism of hormone action. Science 1972; 177:401-408.
13. Huggins C. Endocrine-induced regression of cancers. Science 1967; 156:1050-1054.
14. Kahn SE, Hull RL, Utzschneider KM. Mechanism. linking obesity to insulin resistance and type 2 diabetes. Nature 2006; 444:840-846.
15. Williams DA, Lemke TL. Foye’s Principles of Medicinal Chemistry. 5th edition. 2002. Lippincott Williams & Wilkins, Philadelphia, PA.
16. Reiter RJ. Functional pleiotropy of the neurohormone melatonin: Antioxidant protection and neuroendocrine regulation. Front. Neuroendocrinol. 1995; 16:383-415.
17. Scallet AC. Estrogens: neuroprotective or neurotoxic? Ann. N.Y. Acad. Sci. 1999; 890:121-132.
18. Johnston SR, Dowsett M. Aromatase inhibitors for breast cancer: Lessons from the laboratory. Nat. Rev. Cancer. 2003; 3:821-831.
19. Cohen I, Figer A, Tepper R, Shapira J, Altaras MM, Yigael D, et al. Ovarian overstimulation and cystic formation in premenopausal tamoxifen exposure: comparison between tamoxifen-treated and nontreated breast cancer patients. Gynecol. Oncol. 1999; 72:202-207.
20. Wehling M, Losel R. Non-genomic steroid hormone effects: membrane or intracellular receptors J. Steroid Biochem. Mol. Biol. 2006; 102:180-183.
21. Emilien G, Maloteaux JM, Geurts M, Hoogenberg K, Cragg S. Dopamin. receptors-physiological understanding to therapeutic intervention potential. Pharmacol. Ther. 1999; 84:133-156.
22. Hegde SS, Eglen RM. Peripheral 5-HT4 receptors. FASEB J. 1996; 10:1398-1407.
23. Yao X, Forte JG. Cell biology of acid secretion by the parietal cell. Annu. Rev. Physiol. 2003; 65:103-131.
24. Wong DL. Why is the adrenal adrenergic? Endocr. Pathol. 2003; 14:25-36.
25. Burnstock G. Purinergic signaling. Br. J. Pharmacol. 2006; 147:S172-181.
26. Civelli O, Saito Y, Wang Z, Nothacker HP, Reinscheid RK. Orphan GPCRs and their ligands. Pharmacol. Ther. 2006; 110: 525-532.
27. Shi Y. Orphan nuclear receptors, excellent targets of drug discovery. Comb. Chem. High Throughput Screen. 2006; 9:683-689.
28. Wise A, Jupe SC, Rees S. The identification of ligands at orphan G-protein coupled receptors. Annu. Rev. Pharmacol. Toxicol. 2004; 44:43-66.
29. Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signaling pathway mediated by the nuclear receptor LXR alpha. Nature 1996; 383:728-731.
30. Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, et al. Identification of a nuclear receptor for bile acids. Science 1999; 284:1362-1365.
31. Scanlan TS, Suchland KL, Hart ME, Chiellini G, Huang Y, Kruzich PJ, et al. 3-iodothyronamine is an endogenous and rapid-acting derivative of thyroid hormone. Nat. Med. 2004; 10:638-642.
32. Hart ME, Suchland KL, Miyakawa M, Bunzow JR, Grandy DK, Scanlan TS. Trace amine-associated receptor agonists: synthesis and evaluation of thyronamines and related analogues. J. Med. Chem. 2006; 49:1101-1112.
33. Degim IT, Celebi N. Controlled delivery of peptides and proteins. Curr. Pharm. Des. 2007; 13:99-117.
34. Brown TR. Nonsteroidal selective androgen receptors modulators (SARMs): designer androgens with flexible structures provide clinical promise. Endocrinology 2004; 145:5417-5419.
35. Winneker RC, Fensome A, Wrobel JE, Zhang Z, Zhang P. Nonsteroidal progesterone receptor modulators: structure activity relationships. Semin. Reprod. Med. 2005; 23:46-57.
36. Hruby VJ. Designing peptide receptor agonists and antagonists. Nat. Rev. Drug Discov. 2002; 1:847-858.
37. Kingsmore SF. Multiplexed protein measurement: technologies and applications of protein and antibody arrays. Nat. Rev. Drug Discov. 2006; 5:310-320.
38. Whitty A, Kumaravel G. Between a rock and a hard place? Nat. Chem. Biol. 2006; 2:112-118.
39. Yin H, Hamilton AD. Strategies for targeting protein-protein interactions with synthetic agents. Angew. Chem. Int. Ed. Engl. 2005; 44:4130-4163.
40. Pagliaro L, Felding J, Audouze K, Nielsen SJ, Terry RB, Krog-Jensen C, et al. Emerging classes of protein-protein interaction inhibitors and new tools for their development. Curr. Opin. Chem. Biol. 2004; 8:442-449.
41. Tian SS, Lamb P, King AG, Miller SG, Kessler L, Luengo JI, et al. A small, nonpeptidyl mimic of granulocyte-colony-stimulating factor. Science 1998; 281:257-259.
42. Zhang B, Salituro G, Szalkowski D, Li Z, Zhang Y, Royo I, et al. Discovery of a small molecule insulin mimetic with antidiabetic activity in mice. Science 1999; 284:974-977.
43. Aviezer D, Cotton S, David M, Segev A, Khaselev N, Galili N, et al. Porphyrin analogues as novel antagonists of fibroblast growth factor and vascular endothelial growth factor receptor binding that inhibit endothelial cell proliferation, tumor progression, and metastasis. Cancer. Res. 2000; 60:2973-2980.
44. Arkin MR, Randal M, DeLano WL, Hyde J, Luong TN, Oslob JD, et al. Binding of small molecules to an adaptive protein-protein interface. Proc. Natl. Acad. Sci. U.S.A. 2003; 100:1603-1608.
45. Chockalingam K, Zhao H. Creating new specific ligand-receptor pairs for transgene regulation. Trends Biotechnol. 2005; 23:333-335.
46. Hassan AQ, Koh JT. A functionally orthogonal ligand-receptor pair created by targeting the allosteric mechanism of the thyroid hormone receptor. J. Am. Chem. Soc. 2006; 128:8868-8874.
47. Gallinari P, Lahm A, Koch U, Paolini C, Nardi MC, Roscilli, G, et al. A functionally orthogonal estrogen receptor-based transcription switch specifically induced by a nonsteroidal synthetic ligand. Chem. Biol. 2005; 12:883-893.
48. Kinzel O, Fattori D, Muraglia E, Gallinari P, Nardi MC, Paolini C, et al. A structure-guided approach to an orthogonal estrogen- receptor-based gene switch activated by ligands suitable for in vivo studies. J. Med. Chem. 2006; 49:5404-5407.
49. Gao ZG, Duong HT, Sonina T, Kim SK, Van Rompaey P, Van Calenbergh S, et al. Orthogonal activation of the reengineered A3 adenosine receptor (neoceptor) using tailored nucleoside agonists. J. Med. Chem. 2006; 49:2689-2702.
50. Weatherman RV. Untangling the estrogen receptor web. Nat. Chem. Biol. 2006; 2:175-176.
51. Getting SJ. Targeting melanocortin receptors as potential novel therapeutics. Pharmacol. Ther. 2006; 111:1-15.
52. Holder JR, Haskell-Luevano C. Melanocortin ligands: 30 years of structure-activity relationship (SAR) studies. Med. Res. Rev. 2004; 24:325-356.
53. Al-Obeidi F, Castrucci AM, Hadley ME, Hruby VJ. Potent and prolonged acting cyclic lactam analogues of alpha-melanotropin: design based on molecular dynamics. J. Med. Chem. 1989; 32:2555-2561.
54. Catania A, Gatti S, Colombo G, Lipton JM. Targeting melanocortin receptors as a novel strategy to control inflammation. Pharmacol. Rev. 2004; 56:1-29.
55. Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 1997; 385:165-168.
56. Herpin TF, Yu G, Carlson KE, Morton GC, Wu X, Kang L, et al. Discovery of tyrosine-based potent and selective melanocortin-1 receptor small-molecule agonists with anti-inflammatory properties. J. Med. Chem. 2003; 46:1123-1126.
57. Van der Ploeg LH, Martin WJ, Howard AD, Nargund RP, Austin CP, Guan X, et al. A role for the melanocortin 4 receptor in sexual function. Proc. Natl. Acad. Sci. U.S.A. 2002; 99:11381-11386.
58. Katzenellenbogen JA, O’Malley BW, Katzenellenbogen BS. Tripartite steroid hormone receptor pharmacology - interaction with multiple effector sites as a basis for the cell- and promoter-specific action of these hormones. Mol. Endocrinol. 1996; 10:119-131.
59. Mueller SO, Korach KS. Estrogen receptors and endocrine diseases: Lessons from estrogen receptor knockout mice. Curr. Opin. Pharmacol. 2001; 1:613-619.
60. Ariazi EA, Ariazi JL, Cordera F, Jordan VC. Estrogen receptors as therapeutic targets in breast cancer. Curr. Top. Med. Chem. 2006; 6:181-202.
61. Jordan VC. SERMs: meeting the promise of multifunctional medicines. J. Natl. Cancer Inst. 2007; 99:350-356.
62. Knox AJ, Meegan MJ, Lloyd DG. Estrogen receptors: Molecular interactions, virtual screening and future prospects. Curr. Top. Med. Chem. 2006; 6:217-243.
63. Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: Convergence of genomic and nongenomic actions on target genes. Mol. Endocrinol. 2005; 19:833-842.
64. Arnold LA, Estebanez-Perpina E, Togashi M, Jouravel N, Shelat A, McReynolds AC, et al. Discovery of small molecule inhibitors of the interaction of the thyroid hormone receptor with transcriptional coregulators. J. Biol. Chem. 2005; 280:43048-43055.
65. Navratilova I, Dioszegi M, Myszka DG. Analyzing ligand and small molecule binding activity of solubilized GPCRs using biosensor technology. Anal. Biochem. 2006; 355:132-139.
66. Schumacher C, von Tscharner V. Practical instructions for radioactively labeled ligand receptor binding studies. Anal. Biochem. 1994; 222:262-269.
67. Seethala R, Golla R, Ma Z, Zhang H, O’Malley K, Lippy J, et al. A rapid, homogeneous, fluorescence polarization binding assay for peroxisome proliferator-activated receptors alpha and gamma using a fluorescein-tagged dual PPARalpha/gamma activator. Anal. Biochem. 2007; 363:263-274.
68. Sen S, Jaakola VP, Heimo H, Kivela P, Scheinin M, Lund- strom K, Goldman A. Development of a scintiplate assay for recombinant human alpha(2B)-adrenergic receptor. Anal. Biochem. 2002; 307:280-286.
69. Himes SR, Shannon MF. Assays for transcriptional activity based on the luciferase reporter gene. Methods Mol. Biol. 2000; 130:165-174.
70. Brabek J, Hanks SK. Assaying protein kinase activity. Methods Mol. Biol. 2004; 284:79-90.
71. Post SR, Ostrom RS, Insel PA. Biochemical methods for detection and measurement of cyclic amp and adenylyl cyclase activity. Methods Mol. Biol. 2000; 126:363-374.
72. Simpson AW. Fluorescent measurement of (ca2+)c: basic practical considerations. Methods Mol. Biol. 2006; 312:3-36.
73. Lang P, Yeow K, Nichols A, Scheer A. Cellular imaging in drug discovery. Nat. Rev. Drug Discov. 2006; 5:343-356.
74. Morrison KL, Weiss GA. Combinatorial alanine-scanning. Curr. Opin. Chem. Biol. 2001; 5:302-307.
75. Bernat B, Sun M, Dwyer M, Feldkamp M, Kossiakoff AA. Dissecting the binding energy epitope of a high-affinity variant of human growth hormone: cooperative and additive effects from combining mutations from independently selected phage display mutagenesis libraries. Biochemistry 2004; 43:6076-6084.
76. Pal G, Fong SY, Kossiakoff AA, Sidhu SS. Alternative views of functional protein binding epitopes obtained by combinatorial shotgun scanning mutagenesis. Protein Sci. 2005; 14:2405-2413.
77. Conlon JM. Evolution of the insulin molecule: insights into structure-activity and phylogenetic relationships. Peptides 2001; 22:1183-1193.
78. Hawtin SR, Wesley VJ, Simms J, Argent CC, Latif K, Wheatley M. The N-terminal juxtamembrane segment of the V1a vasopressin receptor provides two independent epitopes required for high-affinity agonist binding and signaling. Mol. Endocrinol. 2005; 19:2871-2881.
79. Yamamoto K, Inaba Y, Yoshimoto N, Choi M, DeLuca HF, Yamada S. 22-alkyl-20-epi-1alpha,25-dihydroxyvitamin D3 compounds of superagonistic activity: syntheses, biological activities and interaction with the receptor. J. Med. Chem. 2007; 50:932-939.
80. Kopchick JJ, Sackmann-Sala L, Ding J. Primer: molecular tools used for the understanding of endocrinology. Nat. Clin. Pract. Endocrinol. Metab. 2007; 3:355-368.
81. Hsueh AJ, Bouchard P, Ben-Shlomo I. Hormonology: a genomic perspective on hormonal research. J. Endocrinol. 2005; 187:333-338.
82. Ojeda SR, Lomniczi A, Mastronardi C, Heger S, Roth C, Parent AS, et al. Minireview: the neuroendocrine regulation of puberty: is the time ripe for a systems biology approach Endocrinology 2006;147:1166-1174.
83. Nicholson RI, Hutcheson IR, Britton D, Knowlden JM, Jones HE, Harper ME, et al. Growth factor signaling networks in breast cancer and resistance to endocrine agents: new therapeutic strategies. J. Steroid Biochem. Mol. Biol. 2005; 93:257-262.
84. Rondinone CM. Adipocyte-derived hormones, cytokines, and mediators. Endocrine 2006; 29:81-90.
Further Reading
Here are a few textbooks that cover a number of the specific hormone signaling systems and protocols for both measuring receptor-ligand interactions and cell-based assays.
Baulieu EE, Kelly PA. Hormones: From Molecules to Disease. 1990. Springer, Berlin.
Bolander FF. Molecular Endocrinology. 2004. Elsevier Academic Press, San Diego, CA.
Dingermann T, Steinhilber D, Folkers G. Molecular Biology in Medicinal Chemistry. 2004. Weinheim, Wiley-VCH.
Nienhaus GU. Protein-Ligand Interactions: Methods and Applications. 2005. Humana Press, Totowa, NJ.
Norman AW, Litwack G, Hormones, 2nd ed. 1997. Academic Press, San Diego, CA.
See Also
Cellular Communication Through Signal Transduction, Chemistry of
Nuclear Receptors
Receptor-Ligand Interactions
Peptidomimetics
Small Molecules to Elucidate GPCR Signaling Pathways