Chemistry for Dummies
Part III. The Mole: The Chemist's Best Friend
Chapter 12. Sour and Bitter: Acids and Bases
In This Chapter
· Discovering the properties of acids and bases
· Finding out about the two acid-base theories
· Differentiating between strong and weak acids and bases
· Understanding indicators
· Taking a look at the pH scale
· Figuring out buffers and antacids
Walk into any kitchen or bathroom, and you’ll find a multitude of acids and bases. Open the refrigerator, and you’ll find soft drinks full of carbonic acid. In the pantry, there’s vinegar and baking soda, an acid and a base. Peek under the sink, and you’ll notice the ammonia and other cleaners, most of which are bases. Check out that can of lye-based drain opener — it’s highly basic. In the medicine cabinet, you’ll find aspirin, an acid, and antacids of all types. Our everyday world is full of acids and bases. And so is the everyday world of the industrial chemist. In this chapter, I cover acids and bases, indicators and pH, and some good basic chemistry.
Properties of Acids and Bases: Macroscopic View
Look at the properties of acids and bases that can be observed in the world around us.
Acids:
ü Taste sour (but remember, in the lab, you test, not taste)
ü Produce a painful sensation on the skin
ü React with certain metals (magnesium, zinc, and iron) to produce hydrogen gas
ü React with limestone and baking soda to produce carbon dioxide
ü React with litmus paper and turn it red
Bases:
ü Taste bitter (again, in the lab, you test, not taste)
ü Feel slippery on the skin
ü React with oils and greases
ü React with litmus paper and turn it blue
ü React with acids to produce a salt and water
Quite a number of acids and bases are found in our everyday life. Tables 12-1 and 12-2 show some common acids and bases found around the home.
Table 12-1. Common Acids Found in the Home
|
Chemical Name |
Formula |
Common Name or Use |
|
hydrochloric acid |
HCl |
muratic acid |
|
acetic acid |
CH3COOH |
vinegar |
|
sulfuric acid |
H2SO4 |
auto battery acid |
|
carbonic acid |
H2CO3 |
carbonated water |
|
boric acid |
H3BO3 |
antiseptic; eye drops |
|
acetylsalicylic acid |
C16H12O6 |
aspirin |
Table 12-2. Common Bases Found in the Home
|
Chemical Name |
Formula |
Common Name or Use |
|
ammonia |
NH3 |
cleaner |
|
sodium hydroxide |
NaOH |
lye |
|
sodium bicarbonate |
NaHCO3 |
baking soda |
|
magnesium hydroxide |
Mg(OH)2 |
milk of magnesia |
|
calcium carbonate |
CaCO3 |
antacid |
|
aluminum hydroxide |
Al(OH)3 |
antacid |
What Do Acids and Bases Look Like? — Microscopic View
If you look at Tables 12-1 and 12-2 closely, you may recognize the fact that all the acids contain hydrogen, while most of the bases contain the hydroxide ion (OH). Two main theories use these facts in their descriptions of acids and bases and their reactions:
ü Arrhenius theory
ü Bronsted-Lowery theory
The Arrhenius theory: Must have water
The Arrhenius theory was the first modern acid-base theory developed. In this theory, an acid is a substance that, when dissolved in water, yields H+ (hydrogen) ions, and a base is a substance that, when dissolved in water, yields OH- (hydroxide) ions. HCl(g) can be considered as a typical Arrhenius acid, because when this gas dissolves in water, it ionizes (forms ions) to give the H+ ion. (Chapter 6 is where you need to go for the riveting details about ions.)
![]()
According to the Arrhenius theory, sodium hydroxide is classified as a base, because when it dissolves, it yields the hydroxide ion:
![]()
Arrhenius also classified the reaction between an acid and base as a neutralization reaction, because if you mix an acidic solution with a basic solution, you end up with a neutral solution composed of water and a salt.
![]()
Look at the ionic form of this equation (the form showing the reaction and production of ions) to see where the water comes from:
![]()
As you can see, the water is formed from combining the hydrogen and hydroxide ions. In fact, the net-ionic equation (the equation showing only those chemical substances that are changed during the reaction) is the same for all Arrhenius acid-base reactions:
![]()
The Arrhenius theory is still used quite a bit. But, like all theories, it has some limitations. For example, look at the gas phase reaction between ammonia and hydrogen chloride gases:
![]()
The two clear, colorless gases mix, and a white solid of ammonium chloride forms. I show the intermediate formation of the ions in the equation so that you can better see what’s actually happening. The HC1 transfers an H+ to the ammonia. That’s basically the same thing that happens in the HCl/NaOH reaction, but the reaction involving the ammonia can’t be classified as an acid-base reaction, because it doesn’t occur in water, and it doesn’t involve the hydroxide ion. But again, the same basic process is taking place in both cases. In order to account for these similarities, a new acid-base theory was developed, the Bronsted-Lowery theory.
The Bronsted-Lowery acid-base theory: Giving and accepting
The Bronsted-Lowery theory attempts to overcome the limitations of the Arrhenius theory by defining an acid as a proton (H+) donor and a base as a proton (H+) acceptor. The base accepts the H+by furnishing a lone pair of electrons for a coordinate-covalent bond, which is a covalent bond (shared pair of electrons) in which one atom furnishes both of the electrons for the bond. Normally, one atom furnishes one electron for the bond and the other atom furnishes the second electron (see Chapter 7). In the coordinate- covalent bond, one atom furnishes both bonding electrons.
Figure 12-1 shows the NH3/HC1 reaction using the electron-dot structures of the reactants and products. (Electron-dot structures are covered in Chapter 7, too.)
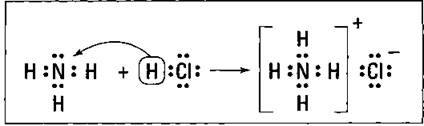
Figure 12-1: Reaction of NH3 with HCl.
HCl is the proton donor, and the acid and ammonia are the proton acceptor, or the base. Ammonia has a lone pair of nonbonding electrons that it can furnish for the coordinate-covalent bond.
I discuss acid-base reactions under the Bronsted-Lowery theory in the section “Give me that proton: Bronsted-Lowery acid-base reactions,” later in this chapter.
Acids to Corrode, Acids to Drink: Strong and Weak Acids and Bases
I want to introduce you to a couple of different categories of acids and bases — strong and weak. However, it’s important to remember that acid-base strength is not the same as concentration. Strength refers to the amount of ionization or breaking apart that a particular acid or base undergoes. Concentration refers to the amount of acid or base that you initially have. You can have a concentrated solution of a weak acid, or a dilute solution of a strong acid, or a concentrated solution of a strong acid or... well, I’m sure you get the idea.
Strong acids
If you dissolve hydrogen chloride gas in water, the HCl reacts with the water molecules and donates a proton to them:
![]()
The H3O+ ion is called the hydronium ion. This reaction goes essentially to completion, meaning the reactants keep creating the product until they’re all used up. In this case, all the HCl ionizes to H3O+ and Cl-, there’s no more HCl present. Acids such as HCl, which ionizes essentially 100 percent in water, are called strong acids. Note that water, in this case, acts as a base, accepting the proton from the hydrogen chloride.
Because strong acids ionize completely, it’s easy to calculate the concentration of the hydronium ion and chloride ion in solution if you know the initial concentration of the strong acid. For example, suppose that you bubble 0.1 moles (see Chapter 10 to get a firm grip on moles) of HCl gas into a liter of water. You can say that the initial concentration of HCl is 0.1 M (0.1 mol/L). M stands for molarity, and mol/L stands for moles of solute per liter. (For a detailed discussion of molarity and other concentration units, see Chapter 11.)
You can represent this 0.1 M concentration for the HCl in this fashion: [HCl] = 0.1. Here, the brackets around the compound indicate molar concentration, or mol/L. Because the HCl completely ionizes, you see from the balanced equation that for every HCl that ionizes, you get one hydronium ion and one chloride ion. So the concentration of ions in that 0.1 M HCl solution is
![]()
This idea is valuable when you calculate the pH of a solution. (And you can do just that in the section “How Acidic Is That Coffee: The pH Scale,” later in this chapter.)
Table 12-3 lists the most common strong acids you’re likely to encounter.
Table 12-3. Common Strong Acids
|
Name |
Formula |
|
Hydrochloric acid |
HCl |
|
Hydrobromic acid |
HBr |
|
Hydroiodic acid |
HI |
|
Nitric acid |
HNO3 |
|
Perchloric acid |
HClO4 |
|
Sulfuric acid (first ionization only) |
H2SO4 |
Sulfuric acid is called a diprotic acid. It can donate 2 protons, but only the first ionization goes 100 percent. The other acids listed in Table 12-3 are monoprotic acids, because they donate only one proton.
Strong bases
You’ll normally see only one strong base, and that’s the hydroxide ion, OH'. Calculating the hydroxide ion concentration is really straightforward. Suppose that you have a 1.5 M (1.5 mol/L) NaOH solution. The sodium hydroxide, a salt, completely dissociates (breaks apart) into ions:
![]()
If you start with 1.5 mol/L NaOH, then you have the same concentration of ions:
![]()
Weak acids
Suppose that you dissolve acetic acid (CH3COOH) in water. It reacts with the water molecules, donating a proton and forming hydronium ions. It also establishes equilibrium, where you have a significant amount of unionized acetic acid. (In reactions that go to completion, the reactants are completely used up creating the products. But in equilibrium systems, two exactly opposite chemical reactions — one on each side of the reaction arrow — are occurring at the same place, at the same time, with the same speed of reaction. For a discussion of equilibrium systems, see Chapter 8.)
TIP. If you want to see whether a person is a chemist, ask him to pronounce unionized. A chemist pronounces it un-ionized, meaning “not ionized.” Everyone else pronounces it union-ized, meaning “being part of a union.”
The acetic acid reaction with water looks like this:
![]()
The acetic acid that you add to the water is only partially ionized. In the case of acetic acid, about 5 percent ionizes, while 95 percent remains in the molecular form. The amount of hydronium ion that you get in solutions of acids that don’t ionize completely is much less than it is with a strong acid. Acids that only partially ionize are called weak acids.
Calculating the hydronium ion concentration in weak acid solutions isn’t as straightforward as it is in strong solutions, because not all of the weak acid that dissolves initially has ionized. In order to calculate the hydronium ion concentration, you must use the equilibrium constant expression for the weak acid. Chapter 8 covers the Keq expression that represents the equilibrium system. For weak acid solutions, you use a modified equilibrium constant expression called the Ka — the acid ionization constant. Take a look at the generalized ionization of some weak acid HA:
![]()
The Ka expression for this weak acid is
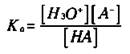
Note that the [HA] represents the molar concentration of HA at equilibrium, not initially. Also, note that the concentration of water doesn’t appear in the Ka expression, because there’s so much that it actually becomes a constant incorporated into the Ka expression.
Now go back to that acetic acid equilibrium. The Ka for acetic acid is 1.8 x 10-5. The Ka expression for the acetic acid ionization is
![]()
You can use this Ka when calculating the hydronium ion concentration in, say, a 2.0 M solution of acetic acid. You know that the initial concentration of acetic acid is 2.0 M. You know that a little bit has ionized, forming a little hydronium ion and acetate ion. You also can see from the balanced reaction that for every hydronium ion that’s formed, an acetate ion is also formed — so their concentrations are the same. You can represent the amount of [H3O+] and [CH3COO ] as x, so
![]()
In order to produce the x amount of hydronium and acetate ion, the same amount of ionizing acetic acid is required. So you can represent the amount of acetic acid remaining at equilibrium as the amount you started with, 2.0 M, minus the amount that ionizes, x:
![]()
For the vast majority of situations, you can say that x is very small in comparison to the initial concentration of the weak acid. So you can say that 2.0 - x is approximately equal to 2.0. This means that you can often approximate the equilibrium concentration of the weak acid with its initial concentration. The equilibrium constant expression now looks like this:
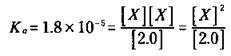
At this point, you can solve for x, which is the [H3O+]:
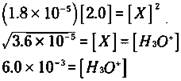
Table 12-3 shows some common strong acids. Most of the other acids you encounter are weak.
TIP. One way to distinguish between strong and weak acids is to look for an acid ionization constant (Ka) value. If the acid has a Ka value, then it’s weak.
Weak bases
Weak bases also react with water to establish an equilibrium system. Ammonia is a typical weak base. It reacts with water to form the ammonium ion and the hydroxide ion:
![]()
Like a weak acid, a weak base is only partially ionized. There’s a modified equilibrium constant expression for weak bases — the Kb. You use it exactly the same way you use the Ka (see “Weak acids” for the details) except you solve for the [OH-].
Give me that proton: Bronsted-Lowery acid-base reactions
With the Arrhenius theory, acid-base reactions are neutralization reactions. With the Bronsted-Lowery theory, acid-base reactions are a competition for a proton. For example, take a look at the reaction of ammonia with water:
![]()
Ammonia is a base (it accepts the proton), and water is an acid (it donates the proton) in the forward (left to right) reaction. But in the reverse reaction (right to left), the ammonium ion is an acid and the hydroxide ion is a base. If water is a stronger acid than the ammonium ion, then there is a relatively large concentration of ammonium and hydroxide ions at equilibrium. If, however, the ammonium ion is a stronger acid, much more ammonia than ammonium ion is present at equilibrium.
Bronsted and Lowery said that an acid reacts with a base to form conjugate acid-base pairs. Conjugate acid base pairs differ by a single H+. NH3 is a base, for example, and NH4+ is its conjugate acid. H2O is an acid in the reaction between ammonia and water, and OH- is its conjugate base. In this reaction, the hydroxide ion is a strong base and ammonia is a weak base, so the equilibrium is shifted to the left — there’s not much hydroxide at equilibrium.
Make up your mind: Amphoteric water
When acetic acid reacts with water, water acts as a base, or a proton acceptor. But in the reaction with ammonia (see the preceding section), water acts as an acid, or a proton donor. Water can act as either an acid or a base, depending on what it’s combined with. Substances that can act as either an acid or a base are called amphoteric. If you put water with an acid, it acts as a base, and vice versa.
But can it react with itself? Yes, it can. Two water molecules can react with each other, with one donating a proton and the other accepting it:
![]()
This reaction is an equilibrium reaction. A modified equilibrium constant, called the Kw (which stands for water dissociation constant) is associated with this reaction. The Kw has a value of 1.0 x 10-14 and has the following form:
![]()
In pure water, the [H3O+] equals the [OH-] from the balanced equation, so [H3O+] = [OH-] = 1.0 x 10-7. The Kw value is a constant. This value allows you to convert from [H+] to [OH-], and vice versa, in any aqueous solution, not just pure water. In aqueous solutions, the hydronium ion and hydroxide ion concentrations are rarely going to be equal. But if you know one of them, the Kw allows you to figure out the other one.
Take a look at the 2.0 M acetic acid solution problem in the section “Weak acids,” earlier in this chapter. You find that the [H3O+] is 6.0 x 10-3. Now you have a way to calculate the [OH-] in the solution by using the Kw relationship:
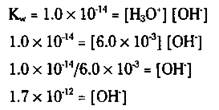
An Old Laxative and Red Cabbage: Acid-Base Indicators
Indicators are substances (organic dyes) that change color in the presence of an acid or base. You may be familiar with an acid-base indicator plant — the hydrangea. If it’s grown in acidic soil, it turns pink; if it’s grown in alkaline soil, it turns blue. Another common substance that acts as a good acid-base indicator is red cabbage. I have my students chop some up and boil it (most of them really love this part). They then take the liquid that is left over and use it to test substances. When mixed with an acid, the liquid turns pink; when mixed with a base, it turns green. In fact, if you take some of this liquid, make it slightly basic, and then exhale your breath into it through a straw, the solution eventually turns blue, indicating that the solution has turned slightly acidic. The carbon dioxide in your breath reacts with the water, forming carbonic acid:
![]()
Carbonated beverages are slightly acidic due to this reaction. Carbon dioxide is injected into the liquid to give it fizz. A little of this carbon dioxide reacts with the water to form carbonic acid. This reaction also explains why rainwater is slightly acidic. It absorbs carbon dioxide from the atmosphere as it falls to earth.
In chemistry, indicators are used to indicate the presence of an acid or base. Chemists have many indicators that change at slightly different pHs. (You’ve probably heard the term pH used in various contexts. Me, I even remember it being used to sell deodorant on TV. If you want to know what it actually stands for, check out the section “How Acidic Is That Coffee: The pH Scale.”) Two indicators are used most often:
ü Litmus paper
ü Phenolphthalein
Good old litmus paper
Litmus is a substance that is extracted from a type of lichen and absorbed into porous paper. (In case you’re scheduled for a hot game of Trivial Pursuit this weekend, a lichen is a plant — found in the Netherlands — that’s made up of an alga and a fungus that are intimately living together and mutually benefiting from the relationship. Sounds kind of sordid to me.) There are three different types of litmus — red, blue, and neutral. Red litmus is used to test for bases, and blue litmus is used to test for acids, while neutral litmus can be used to test for both. If a solution is acidic, both blue and neutral litmus will turn red. If a solution is basic, both red and neutral litmus will turn blue. Litmus paper is a good, quick test for acids and bases. And you don’t have to put up with the smell of boiling cabbage.
Phenolphthalein: Helps keep you regular
Phenolphthalein (pronounced fe-nul-tha-Leen) is another commonly used indicator. Until a few years ago, phenolphthalein was used as the active ingredient in a popular laxative. In fact, I used to extract the phenolphthalein from the laxative by soaking it in either rubbing alcohol or gin (being careful not to drink it). I’d then use this solution as an indicator.
Phenolphthalein is clear and colorless in an acid solution and pink in a basic solution. It’s commonly used in a procedure called a titration, where the concentration of an acid or base is determined by its reaction with a base or acid of known concentration.
Suppose, for example, that you want to determine the molar concentration of an HCl solution. First, you place a known volume (say, 25.00 milliliters measured accurately with a pipette) in an Erlenmeyer flask (that’s just a flat- bottomed, conical-shaped flask) and add a couple drops of phenolphthalein solution. Because you’re adding the indicator to an acidic solution, the solution in the flask remains clear and colorless. You then add small amounts of a standardized sodium hydroxide solution of known molarity (for example, 0.100 M) with a buret. (A buret is a graduated glass tube with a small opening and a stopcock, which helps you measure precise volumes of solution.) You keep adding base until the solution turns the faintest shade of pink detectable. I call this the endpoint of the titration, the point in which the acid has been exactly neutralized by the base. Figure 12-2 shows the titration setup.
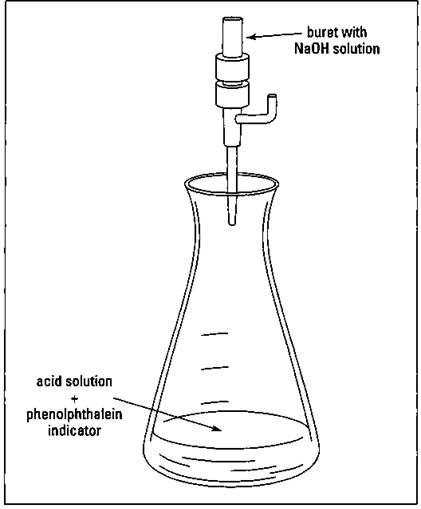
Figure 12-2: Titration of an acid with a base.
Suppose that it takes 35.50 milliliters of the 0.100 M NaOH to reach the endpoint of the titration of the 25.00 milliliters of the HCl solution. Here’s the reaction:
![]()
From the balanced equation, you can see that the acid and base react in a 1:1 mole ratio. So if you can calculate the moles of bases added, you’ll also know the number of moles of HCl present. Knowing the volume of the acid solution then allows you to calculate the molarity (note that you convert the milliliters to liters so that your units cancel nicely):
![]()
The titration of a base with a standard acid solution (one of known concentration) can be calculated in exactly the same way, except the endpoint is the first disappearance of the pink color.
How Acidic Is That Coffee: The pH Scale
The amount of acidity in a solution is related to the concentration of the hydronium ion in the solution. The more acidic the solution is, the larger the concentration of the hydronium ion. In other words, a solution in which the [H3O+] equals 1.0 x 10'2 is more acidic than a solution in which the [H3O+] equals 1.0 x 10-7. The pH scale, a scale based on the [H3O+], was developed to more easily tell, at a glance, the relative acidity of a solution. pH is defined as the negative logarithm (abbreviated as log) of the [H3O+]. Mathematically, it looks like this:
![]()
Based on the water dissociation constant, Kw (see “Make up your mind: Amphoteric water,” earlier in this chapter), in pure water the [H3O+] equals 1.0 x 10-7. Using this mathematical relationship, you can calculate the pH of pure water:
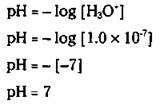
The pH of pure water is 7. Chemists call this point on the pH scale neutral. A solution is called acidic if it has a larger [H3O+] than water and a smaller pH value than 7. A basic solution has a smaller [H3O+] than water and a larger pH value than 7.
The pH scale really has no end. You can have a solution of pH that registers less than 0. (A 10 M HCl solution, for example, has a pH of -1.) However, the 0 to 14 range is a convenient range to use for weak acids and bases and for dilute solutions of strong acids and bases. Figure 12-3 shows the pH scale.
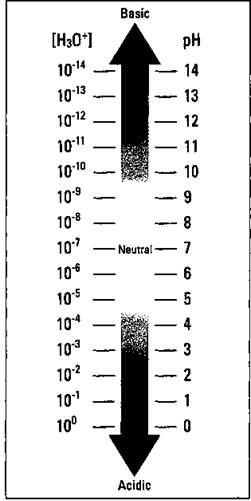
Figure 12-3: The pH scale.
The [H3O+] of a 2.0 M acetic acid solution is 6.0 x 10-3. Looking at the pH scale, you see that this solution is acidic. Now calculate the pH of this solution:
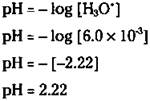
In the section “Make up your mind: Amphoteric water,” I explain that the Kw expression enables you to calculate the [H3O+] if you have the [OH-]. Another equation, called the pOH, can be useful in calculating the pH of a solution. The pOH is the negative logarithm of the [OH-]. You can calculate the pOH of a solution just like the pH by taking the negative log of the hydroxide ion concentration. If you use the Kw expression and take the negative log of both sides, you get 14 = pH + pOH. This equation makes it easy to go from pOH to pH.
Just as you can you convert from [H3O+] to pH, you can also go from pH to [H3O+]. To do this, you use what’s called the antilog relationship, which is
![]()
Human blood, for example, has a pH of 7.3. Here’s how you calculate the [H3O+] from the pH of blood:
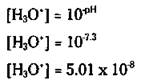
The same procedure can be used to calculate the [OH-] from the pOH.
Substances commonly found in our surroundings cover a wide range of pH values. Table 12-4 lists some common substances and their pH values.
Table 12-4. Average pH Values of Some Common Substances
|
Substance |
PH |
||
|
Oven cleaner |
13.8 |
||
|
Hair remover |
12.8 |
||
|
Household ammonia |
11.0 |
||
|
Milk of magnesia |
10.5 |
||
|
Chlorine bleach |
9.5 |
||
|
Seawater |
8.0 |
||
|
Human blood |
7.3 |
||
|
Pure water |
7.0 |
||
|
Milk |
6.5 |
||
|
Black coffee |
5.5 |
||
|
Soft drinks |
3.5 |
||
|
Aspirin |
2.9 |
||
|
Vinegar |
2.8 |
||
|
Lemon juice |
2.3 |
|
|
|
Auto battery acid |
0.8 |
|
|
Human blood has a pH of around 7.3. There’s a narrow range in which blood pH can change and still sustain life, about +/- 0.2 pH units. Many things in our environment, such as foods and hyperventilation, can act to change the pH of our blood. Buffers help to regulate blood pH and keep it in the 7.1 to 7.5 range.
Buffers: Controlling pH
Buffers, or buffer solutions as they’re sometimes called, resist a change in pH caused by the addition of acids or bases. Obviously, the buffer solution must contain something that reacts with an acid — a base. Something else in the buffer solution reacts with a base — an acid. There are, in general, two types of buffers:
ü Mixtures of weak acids and bases
ü Amphoteric species
The mixtures of weak acids and bases may be conjugate acid-base pairs (such as H2CO3/HCO3-) or nonconjugate acid-base pairs (such as NH4+/CH3COO-). (For more info about conjugate acid-base pairs, see “Give me that proton: Bronsted-Lowery acid-base reactions,” earlier in this chapter.)
In the body, conjugate acid-base pairs are more common. In the blood, for example, the carbonic acid/bicarbonate pair helps to control the pH. This buffer can be overcome, though, and some potentially dangerous situations can arise. If a person exercises strenuously, lactic acid from the muscles is released into the bloodstream. If there’s not enough bicarbonate ion to neutralize the lactic acid, the blood pH drops, and the person is said to be in acidosis. Diabetes may also cause acidosis. On the other hand, if a person hyperventilates (breathes too fast), she breathes out too much carbon dioxide. The carbonic acid level in the blood is reduced, causing the blood to become too basic. This condition, called alkalosis, can be very serious.
Amphoteric species may also act as buffers by reacting with an acid or a base. (For an example of an amphoteric species, see “Make up your mind: Amphoteric water,” earlier in this chapter) The bicarbonate ion (HCO3-) and the monohydrogen phosphate ion (HPO4-2) are amphoteric species that neutralize both acids and bases. Both of these ions are also important in controlling the blood’s pH.
Antacids: Good, Basic Chemistry
Go to any drugstore or grocery store and look at the shelves upon shelves of antacids. They represent acid-base chemistry in action!
The stomach secretes hydrochloric acid in order to activate certain enzymes (biological catalysts) in the digestion process. But sometimes the stomach produces too much acid, or the acid makes its way up into the esophagus (leading to heartburn), making it necessary to neutralize the excess acid with — you guessed it — a base. The basic formulations that are sold to neutralize this acid are called antacids. Antacids include the following compounds as active ingredients:
ü Bicarbonates — NaHCO3 and KHCO3
ü Carbonates — CaCO3 and MgCO3
ü Hydroxides — Al(OH)3 and Mg(OH)2
Acids with bad press: An introduction to acid rain
Over the past few years, acid rain has emerged as a great environmental problem. Natural rainwater is somewhat acidic (around pH 5.6) due to the absorption of carbon dioxide from the atmosphere and the creation of carbonic acid. However, when acid rain is mentioned in the press, it usually refers to rain in the pH 3.to 3.5 range.
The two major causes of acid rain are automotive and industrial pollution. In the automobile's internal combustion engine, nitrogen in the air is oxidized to various oxides of nitrogen. These nitrogen oxides, when released into the atmosphere, react with water vapor to form nitric acid HNO3).
In fossil fuel power plants, oxides of sulfur are formed from the burning of the sulfur impurities commonly found in coal and petroleum. These oxides of sulfur, if released into the atmosphere, combine with water vapor to form both sulfuric and sulfurous acids (H2SO4 and H2SO3). Oxides of nitrogen are also produced in these power plants.
These acids fall to earth in the rain and cause a multitude of problems. They dissolve the calcium carbonate of marble statues and monuments, They decrease the pH of lake water to such a degree that fish can no longer live in the lakes. They cause whole forests to die or become stunted. They react with the metals in cars and buildings.
Industrial controls have been somewhat effective in reducing the problem, but ifs still a major environmental issue. (See Chapter 18 for more info about acid rain.)
Trying to select the “best” antacid for occasional use can be complicated. Certainly price is a factor, but the chemical nature of the bases can also be a factor. For example, individuals with high blood pressure may want to avoid antacids containing sodium bicarbonate because the sodium ion tends to increase blood pressure. Individuals concerned about loss of calcium from the bones, or osteoporosis, may want to use an antacid containing calcium carbonate. However, both calcium carbonate and aluminum hydroxide can cause constipation if used in large doses. On the other hand, large doses of both magnesium carbonate and magnesium hydroxide can act as laxatives. Selecting an antacid can really be a balancing act!