Chemistry for Dummies
Part IV. Chemistry in Everyday Life: Benefits and Problems
Chapter 15. Petroleum: Chemicals for Burning or Building
In This Chapter
· Discovering how petroleum is refined
· Taking a look at gasoline
Petroleum is the basis of our modem society. Our automobiles run on w gasoline, which is produced in part from petroleum, and many of our homes are heated with petroleum. It provides the feedstock for the expansive petrochemical industry. It’s used to make plastics, paints, medicines, textiles, herbicides, and pesticides. The list is almost endless. Every year, the United States consumes over six billion barrels of petroleum. States and whole nations have risen to prosperity thanks to petroleum.
In this chapter, I show you how petroleum is refined and converted into useful products. I concentrate on the production of gasoline, because it’s one of the most economically important uses of petroleum. I show you some of the problems that have been caused by our reliance on the internal combustion engine. This is one chapter in which I can talk crude — oil, that is.
Don't Be Crude, Get Refined
Petroleum, or crude oil (sometimes referred to as “Black Gold” or “Texas Tea”), as it comes out of the ground, is a complex mixture of hydrocarbons (see Chapter 14) of varying molecular weights. The lighter hydrocarbons are gases dissolved in the liquid mixture, while the heavier hydrocarbons are higher molecular weight solids that are also dissolved in the liquid mixture. The mixture was formed from decaying animal and plant material that was in the earth’s crust for a very long time. Because it takes an extremely long time for petroleum to form (many millions of years), it’s called a nonrenewable resource.
Before the hydrocarbon mixture can really be of much economic value, it must be refined, freed from impurities or unwanted material. The mixture is separated into groups of hydrocarbons, and in some cases, the molecular structure of the hydrocarbons is changed. The refining process occurs at a plant called a refinery, which produces the refined mixtures and individual compounds that are used for gasoline and feedstock for the vast petrochemical industry. A number of processes occur at the refinery, starting with the fractional distillation of the crude petroleum.
Fractional distillation: Separating chemicals
You’ve probably simmered a liquid in a covered pot on the stove. And you’ve probably noticed that when you remove the lid, water is on the inside of the lid. The heat has caused the water to evaporate from the liquid, and the vapors have condensed back into a liquid on the inside of the cooler lid. This is the most basic example of a process called distillation.
In the laboratory, you can take a mixture of liquids and carefully heat them. The liquid with the lowest boiling point boils first. You can then condense this vapor back to a liquid and collect it. The substance with the next highest boiling point then begins to boil, and so on. You can use this process of distillation as a means for separating the components of a mixture and purifying them. Distillation is an important procedure in organic chemistry, and it’s the first step in the refining process. The distillation process that’s commonly used in the refining industry is called fractional distillation. In this process, the petroleum mixture is heated and different fractions (groups of hydrocarbons with similar boiling points) are collected. Figure 15-1 shows the fractional distillation of crude oil.
The crude oil is brought into the refinery by pipeline and is initially heated and vaporized in a furnace. The hot vapors are then allowed to enter a huge distillation column, called a fractional distillation tower. The vapors containing the lightest molecular weight hydrocarbons rise to the top of the tower. The higher the molecular weight of the hydrocarbons, the lower the level to which they rise. The various fractions are then collected as each hydrocarbon reaches its distinct boiling point. The hydrocarbons within a fraction are all somewhat similar in size and complexity and can be used for the same purposes in the chemical industry. Six fractions are commonly collected:
ü The first fraction is composed of the lightest hydrocarbons, which are gases with a boiling point of less than 40 degrees Celsius. A major component of this fraction is methane (CH4), a gas that’s sometimes called “marsh gas” because it was first found in marshes. Its primary use is as a fuel, natural gas, because it’s a very clean-burning gas. Propane (C3H8) and butane (C4H10) are also found in this fraction. These two gases are normally collected and put under pressure, a process that causes them to liquefy. They can then be transported by truck as liquefied petroleum (LP) gas and used as fuel. This fraction is also used as starting materials in the synthesis of plastics.
ü The second fraction is composed of hydrocarbons of C5H12 (pentane) to C12H26 (dodecane), with boiling points below the 200 degrees Celsius range. This fraction is commonly called natural gasoline or straight-run gasoline, because it can be used in automobile engines with little additional refining. With each barrel (42 gallons) of crude oil that starts out in the tower, less than a quarter of a barrel of straight-run gasoline is produced.
ü The third fraction is composed of hydrocarbons of 12 to 16 carbon atoms in the boiling range of 150 to 275 degrees Celsius. This fraction is used as kerosene and jet fuel. In the next section, I tell you how this fraction is also used to make additional gasoline.
ü The fourth fraction is composed of hydrocarbons in the 12 to 20 carbon-atom chains, with a boiling range of 250 to 400 degrees Celsius. This fraction is used for heating oil and diesel fuel. Again, it can be used in the production of additional gasoline.
ü The fifth fraction is composed of hydrocarbons in the 20 to 36 carbon- atom range, with boiling points of 350 to 550 degrees Celsius. They’re used as greases, lubricating oils, and paraffin-based waxes.
ü The sixth fraction is composed of the residue of semisolid and solid materials that has a boiling point well above 550 degrees Celcius. It’s used as asphalt and tar.
This cracks me up: Catalytic cracking
A barrel of crude oil yields a wide variety of products, but they don’t all have the same value to us. Gasoline is the product of petroleum that’s in the highest demand. The straight-run gasoline fraction that comes directly from the crude oil can’t keep pace with the demand for gasoline.
With the high demand for gasoline, somebody got the bright idea that if you take a fraction of higher molecular weight hydrocarbons and break it down into smaller chains, the lower molecular weight hydrocarbons can be used for gasoline. The idea of catalytic cracking was born.
In a catalytic cracking plant (“cat crackers,” as they’re called in Texas), fractions in the C12 to C20 range are heated in the absence of air with a catalyst. This process causes the long alkanes (compounds of carbon and hydrogen with only carbon-to-carbon single bonds, which are covered in glorious detail in Chapter 14) to break apart into smaller alkanes and alkenes (hydrocarbons with at least one carbon-to-carbon double bond, covered in equally glorious detail in Chapter 14).
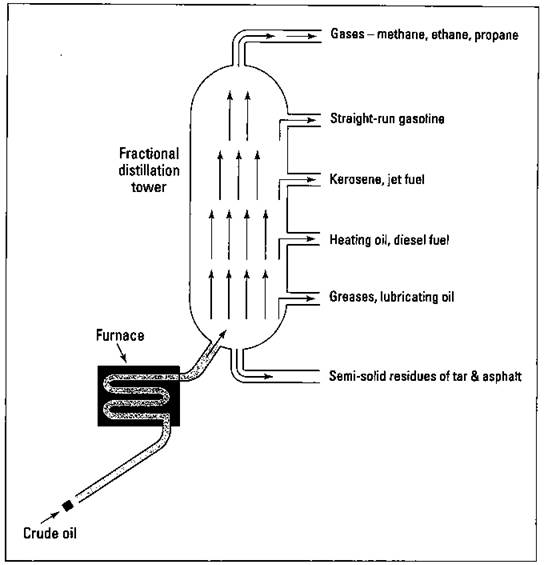
Figure 15-1: Fractional distillation of petroleum.
For example, suppose that you take C20H42 and “crack it”:
![]()
This process yields hydrocarbons that are useful in the production of gasoline. In fact, the double bonds actually give it a higher octane rating, as I explain in “The Gasoline Story,” later in this chapter.
Catalytic cracking is done on the fraction that’s used for kerosene and jet fuel. But in order to produce even more gasoline, catalytic cracking is also done on the fraction used for heating oil. Using this fraction can present a problem, though, if a severe winter hits and the demand for heating oil skyrockets. Oil companies watch the long-range weather forecasts closely. In the summer, when the demand for gasoline is high, fractions that can be used for heating oil are converted to gasoline to meet the demand. Then, as fall arrives, refineries shift their production schedule somewhat. They reduce the amount of gasoline they produce and increase the amount of heating oil so that the winter demand for heating oil can be met. But the refineries don’t want to overproduce heating oil and have to store large amounts, so they try to second-guess the weather to develop a supply that will meet the demand. It’s a real balancing act.
Moving molecular parts around: Catalytic reforming
As the internal combustion engine gained popularity as a mode of transportation, chemists noted that if the gasoline contained only straight-chained hydrocarbons, it didn’t burn properly; it had a tendency to knock or ping. They found that hydrocarbons with branched structures burned much better. In order to increase the amount of branching in the petroleum hydrocarbon fraction being used for gasoline, a process called catalytic reforming was developed. In this process, the hydrocarbon vapors are passed over a metal catalyst such as platinum, and the molecule is rearranged into one with a branched structure or even a cyclic structure. Figure 15-2 shows the catalytic reforming of n-hexane to 2-methylpentane and to cyclohexane.
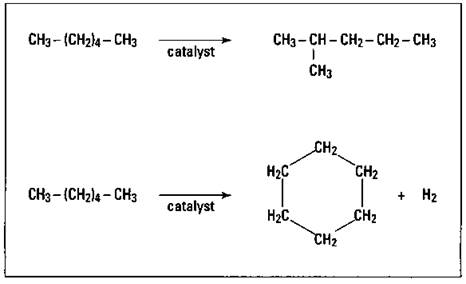
Figure 15-2: Catalytic reforming of n-hexane.
This same process is used extensively to produce benzene and other aromatic compounds for use in the manufacture of plastics, medicines, and synthetic materials. (For a discussion of aromatic compounds, as well as branched and cyclic structures, see Chapter 14. What a treasure trove it is.)
The Gasoline Story
In order for you to better understand the properties of gasoline, I want to tell you a little about how gasoline is reacted in an internal combustion engine. The gasoline is mixed with air (a mixture of nitrogen, oxygen, and so on) and injected into the cylinder as the piston moves to the bottom of the cylinder. The piston then begins to move upward, compressing the gasoline-air mixture. At just the right moment, the spark plug fires, igniting the mixture. The hydrocarbons react with the oxygen in the cylinder, producing water vapor, carbon dioxide, and, unfortunately, large amounts of carbon monoxide.
This reaction is an example of converting the potential energy contained in the hydrocarbon bonds to the kinetic energy of the hot gas molecules. The increase in the number of gas molecules boosts the pressure tremendously, shoving the piston down. The linear motion is then converted to a rotary motion, which powers the wheels. And off you go!
The gasoline-air mixture must ignite at exactly the right moment in order for the engine to operate properly. This process is largely a property of the gasoline and not the engine itself (assuming the timing is set correctly, the spark plugs are good, the compression ratio is okay, and so on). The volatility of the hydrocarbon fuel (that is, how easily it’s converted into a vapor) is important. Volatility is related to the boiling point of the hydrocarbon. In fact, manufacturers blend (adjust the hydrocarbon mixture) their gasoline to match the climate. (They don’t do that in the part of Texas I live in — it’s summer almost year-round.) Winter gas is more volatile than summer gas. Some fuels are prone to produce knocking or pinging in an engine. This propensity to cause knocking or pinging may be a result of preignition, where the igniting of the gasoline occurs before the compression of the fuel-air mixture is complete, or spotty ignition, where combustion starts taking place at a number of sites in the cylinder instead of right around the spark plug electrode. Again, this is a property of the gasoline and not the engine. The energy content of the fuel is important, but how efficiently it burns in the cylinder is just as important. The octane rating scale was developed to rate the burning characteristics of a gasoline.
How good is your gas: Octane ratings
In the early stages of the development of the internal combustion engine, scientists and engineers found that certain hydrocarbons burned well in an internal combustion engine. They also found that certain hydrocarbons did not burn well in these engines. A hydrocarbon that did not burn well was n-heptane (straight-chained heptane). However, 2,2,4-trimethylpentane (commonly called isooctane) had excellent burning characteristics. These two compounds were chosen to define the octane rating scale. The hydrocarbon n-heptane was assigned an octane rating of zero, while isooctane was given a value of 100. Blends of gasoline are then burned in a standard engine and are rated according to this scale. For example, if a particular gasoline blend burns 90 percent as well as isooctane, then it’s assigned an octane value of 90. Figure 15-3 shows the octane scale and the octane values of certain pure compounds.
Look carefully at Figure 15-3. A couple of things are useful to note in terms of octane rating and chemical structure. The n-pentene has an octane value of 62. Its octane value can be increased to 91 by introducing a double bond (making it 1-pentene) and making it an unsaturated compound. The octane value increases by almost 30 points with the introduction of the double bond.
The process of catalytic reforming introduces chains, and catalytic cracking introduces double bonds. Not only do these two processes increase the amount of gasoline that’s produced, but they also improve the quality of the gasoline’s burning characteristics. Also notice that benzene, an aromatic compound, has an octane value of 106. Its burning characteristics are better than isooctane. Other substituted aromatic compounds have octane ratings of almost 120. However, benzene and some related compounds are health hazards, so they’re not used.
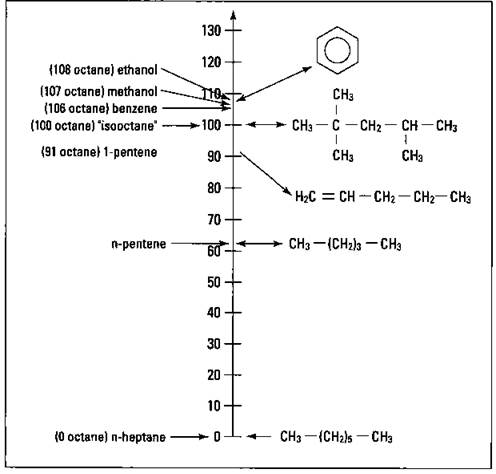
Figure 15-3: The octane rating scale.
The octane rating that is posted on gas pumps is really an average of two kinds of ratings. The Research octane rating (R) relates to the burning characteristics of the fuel in a cold engine. The Motoring octane value (M) refers to how the fuel behaves while you’re cruising down the interstate. If you average R and M — (R+M)/2 — you get the posted octane rating.
Additives: Put the lead in, yet the lead out
The first gasoline engines had a compression ratio that was much lower than today’s automobile engines, and they required lower octane gas. However, as engines became more powerful, gasoline with a higher octane rating was required. Catalytic cracking and reforming added significant cost to the gasoline. The search was on for something cheap that could be added to gasoline to effectively increase the octane rating. The substance tetraethyllead, or TEL, was found.
In the early 1920s, scientists discovered that adding a little bit of TEL to gasoline (1 milliliter per liter of gasoline) increased the octane rating by 10 to 15 points.
Tetraethyllead is basically a lead atom with four ethyl groups attached to it. Figure 15-4 shows the structure of TEL.
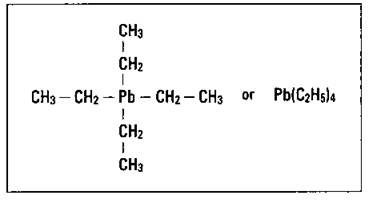
Figure 15-4: The composition of tetraethyllead.
TEL was quite effective as an additive to increase the octane rating and prevent engine knocking. It was used for many years. However, the Clean Air Act of 1970 indirectly did it in.
Oops! We’re polluting the air
Hydrocarbon fuel burns in the cylinders of internal combustion engines. During this process, not all of the hydrocarbon molecules are converted to water and CO/CO2. Before the Clean Air Act of 1970 came into play, unburned hydrocarbons and oxides of both sulfur and nitrogen were being released into the environment from automobiles (along with lead from the TEL, which was later discovered to be very toxic). These gaseous pollutants dramatically increased the amount and severity of air pollution and gave rise to health hazards such as photochemical smog. (For a more complete discussion of air pollution, see Chapter 18.)
Bring on the catalytic converter
In the United States, The Clean Air Act of 1970 mandated the reduction of automotive pollutant emissions. The most effective way to accomplish the reduction of emissions was through the use of a catalytic converter. It’s shaped like a muffler and connected to the exhaust system of an automobile. It has a solid catalyst, either palladium or platinum, inside. When the exhaust gases pass over the catalyst, the catalytic converter helps to complete the oxidation of the hydrocarbons and carbon monoxide to carbon dioxide and water. In other words, it helps to change the harmful gases from gasoline to mostly harmless products.
Lose the lead
The catalytic converter worked well at reducing the automotive emissions as long as there was no lead in the fuel. But if leaded gasoline was used, the lead vapor in the exhaust gases would coat the catalyst, rendering it useless. So there was a big push by the government and environmental groups to “get the lead out.” Now it’s very difficult to find leaded gasoline in the United States, although it’s still available in some foreign countries.
With TEL no longer available as an octane booster, chemists tried to find other compounds to replace it. Aromatic compounds were effective in enhancing the octane value, but they were discovered to be serious health hazards. Recently, methyl alcohol, tert-butyl alcohol, and methyl tert-butyl ether (MTBE) have been used as octane boosters.
MTBE (see Figure 15-5) showed great promise because it not only boosted the octane rating but also acted as an oxygenate, a compound containing oxygen that increases the efficiency of the complete hydrocarbon combustion. But it has already been removed from gasoline due to increasing evidence that it’s related to respiratory illnesses and possible cancers in humans. As for the other compounds, although none of them are as effective as TEL, the partial redesign of the internal combustion engine has allowed the use of slightly lower octane fuels.
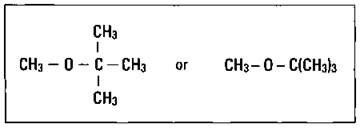
Figure 15-5: Methyl tert-butyl ether (MTBE).