Chemistry for Dummies
Part II. Blessed Be the Bonds That Tie
Chapter 8. Chemical Cooking: Chemical Reactions
In This Chapter
· Differentiating between reactants and products
· Finding out how reactions occur
· Taking a look at the different types of reactions
· Understanding how to balance reactions
· Figuring out chemical equilibrium
· Checking out speeds of reaction
Chemists do a lot of things: They measure the physical properties of substances; they analyze mixtures to find out what they’re composed of; and they make new substances. The process of making chemical compounds is called synthesis. Synthesis depends on chemical reactions. I always thought that it’d be neat to be a synthetic organic chemist and work on the creation of new and potentially important compounds. I can just imagine the thrill of working for months, or even years, and finally ending up with a little pile of “stuff” that nobody in the world has ever seen. Hey, I am a nerd, after all!
In this chapter, I discuss chemical reactions — how they occur and how to write a balanced chemical equation. I also tell you about chemical equilibrium and explain why chemists can’t get the amount of product out of a reaction that they thought they could. And finally, I discuss the speed of reaction and why you shouldn’t leave that turkey sitting out on the table after finishing your Thanksgiving feast.
What you Have and What You’ll Get: Reactants and Products
In a chemical reaction, substances (elements and/or compounds) are changed into other substances (compounds and/or elements). You can’t change one element into another in a chemical reaction — that happens in nuclear reactions, as I describe in Chapter 5. Instead, you create a new substance with chemical reactions.
A number of clues show that a chemical reaction has taken place — something new is visibly produced, a gas is created, heat is given off or taken in, and so on. The chemical substances that are eventually changed are called the reactants, and the new substances that are formed are called the products. Chemical equations show the reactants and products, as well as other factors such as energy changes, catalysts, and so on. With these equations, an arrow is used to indicate that a chemical reaction has taken place. In general terms, a chemical reaction follows this format:
Reactants → Products
For example, take a look at the reaction that occurs when you light your natural gas range in order to fry your breakfast eggs. Methane (natural gas) reacts with the oxygen in the atmosphere to produce carbon dioxide and water vapor. (If your burner isn’t properly adjusted to give that nice blue flame, you may also get a significant amount of carbon monoxide along with carbon dioxide.) The chemical equation that represents this reaction is written like this:
![]()
You can read the equation like this: One molecule of methane gas, CH4(g), reacts with two molecules of oxygen gas, O2(g), to form one molecule of carbon dioxide gas, CO2(g), and two molecules of water vapor, H2O(g). The 2 in front of the oxygen gas and the 2 in front of the water vapor are called the reaction coefficients. They indicate the number of each chemical species that reacts or is formed. I show you how to figure out the value of the coefficients in the section “Balancing Chemical Reactions,” later in the chapter.
Methane and oxygen (oxygen is a diatomic — two-atom — element) are the reactants, while carbon dioxide and water are the products. All the reactants and products are gases (indicated by the g's in parentheses).
In this reaction, all reactants and products are invisible. The heat being evolved is the clue that tells you a reaction is taking place. By the way, this is a good example of an exothermic reaction, a reaction in which heat is given off. A lot of reactions are exothermic. Some reactions, however, absorb energy rather than release it. These reactions are called endothermic reactions. Cooking involves a lot of endothermic reactions — frying those eggs, for example. You can’t just break the shells and let the eggs lie on the pan and then expect the myriad chemical reactions to take place without heating the pan (except when you’re outside in Texas during August; there, the sun will heat the pan just fine).
Thinking about cooking those eggs brings to mind another issue about exothermic reactions. You have to ignite the methane coming out of the burners with a match, lighter, pilot light, or built-in electric igniter. In other words, you have to put in a little energy to get the reaction going. The energy you have to supply to get a reaction going is called the activation energy of the reaction. (In the next section, I show you that there’s also an activation energy associated with endothermic reactions, but it isn’t nearly as obvious.)
But what really happens at the molecular level when the methane and oxygen react? Divert thine eyes to the very next section to find out.
How Do Reactions Occur? Collision Theory
In order for a chemical reaction to take place, the reactants must collide. It’s like playing pool. In order to drop the 8-ball into the corner pocket, you must hit it with the cue ball. This collision transfers kinetic energy (energy of motion) from one belli to the other, sending the second ball (hopefully) toward the pocket. The collision between the molecules provides the energy needed to break the necessary bonds so that new bonds can be formed.
But wait a minute. When you play pool, not every shot you make causes a ball to go into the pocket. Sometimes you don’t hit the ball hard enough, and you don’t transfer enough energy to get the ball to the pocket. This is also true with molecular collisions and reactions. Sometimes, even if there is a collision, not enough kinetic energy is available to be transferred — the molecules aren’t moving fast enough. You can help the situation somewhat by heating the mixture of reactants. The temperature is a measure of the average kinetic energy of the molecules; raising the temperature increases the kinetic energy available to break bonds during collisions.
Sometimes, even if you hit the ball hard enough, it doesn’t go into the pocket because you didn’t hit it in the right spot. The same is true during a molecular collision. The molecules must collide in the right orientation, or hit at the right spot, in order for the reaction to occur.
Here’s an example: Suppose you have an equation showing molecule AS reacting with C to form C-A and B, like this:
![]()
The way this equation is written, the reaction requires that reactant C collide with A-B on the A end of the molecule. (You know this because the product side shows C hooked up with A — C-A) If it hits the B end, nothing will happen. The A end of this hypothetical molecule is called the reactive site, the place on the molecule that the collision must take place in order for the reaction to occur. If C collides at the A end of the molecule, then there’s a chance that enough energy can be transferred to break the AS bond. After the A-B bond is broken, the C-A bond can be formed. The equation for this reaction process can be shown in this way (I show the breaking of the AB bond and the forming of the CA bond as “squiggly” bonds):
![]()
So in order for this reaction to occur, there must be a collision between C and A-B at the reactive site. The collision between C and A-B has to transfer enough energy to break the A-B bond, allowing the C-A bond to form.
REMEMBER. Energy is required to break a bond between atoms.
Note that this example is a simple one. I’ve assumed that only one collision is needed, making this a one-step reaction. Many reactions are one-step, but many others require several steps in going from reactants to final products. In the process, several compounds may be formed that react with each other to give the final products. These compounds are called intermediates. They’re shown in the reaction mechanism, the series of steps that the reaction goes through in going from reactants to products. But in this chapter, I keep it simple and pretty much limit my discussion to one-step reactions.
An exothermic example
Imagine that the hypothetical reaction ![]() is exothermic — a reaction in which heat is given off (released) when going from reactants to products. The reactants start off at a higher energy state than the products, so energy is released in going from reactants to products. Figure 8-1 shows an energy diagram of this reaction.
is exothermic — a reaction in which heat is given off (released) when going from reactants to products. The reactants start off at a higher energy state than the products, so energy is released in going from reactants to products. Figure 8-1 shows an energy diagram of this reaction.
In Figure 8-1, Ea is the activation energy for the reaction — the energy that you have to put in to get the reaction going. I show the collision of C and A-B with the breaking of the A-B bond and the forming of the C-A bond at the top of an activation energy hill. This grouping of reactants at the top of the activation energy hill is sometimes called the transition state of the reaction. As I show in Figure 8-1, the difference in the energy level of the reactants and the energy level of the products is the amount of energy (heat) that is released in the reaction.
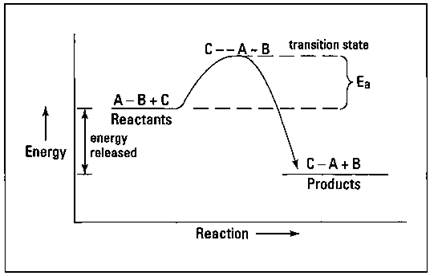
Figure 8-1: Exothermic reaction of ![]() .
.
An endothermic example
Suppose that the hypothetical reaction ![]() is endothermic — a reaction in which heat is absorbed in going from reactants to products — so the reactants are at a lower energy state than the products. Figure 8-2 shows an energy diagram of this reaction.
is endothermic — a reaction in which heat is absorbed in going from reactants to products — so the reactants are at a lower energy state than the products. Figure 8-2 shows an energy diagram of this reaction.
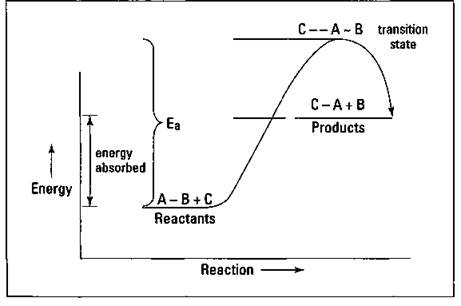
Figure 8-2: Endothermic reaction of ![]() .
.
Just as with the exothermic-reaction energy diagram shown in Figure 8-1, this diagram shows that an activation energy is associated with the reaction (represented by Eg). In going from reactants to products, you have to put in more energy initially to get the reaction started, and then you get that energy back out as the reaction proceeds. Notice that the transition state appears at the top of the activation energy hill — just like in the exothermic-reaction energy diagram. The difference is that, in going from reactants to products, energy (heat) must be absorbed in the endothermic example.
What Kind of Reaction Do You Think I Am?
Several general types of chemical reactions can occur based on what happens when going from reactants to products. The more common reactions are
ü Combination
ü Decomposition
ü Single displacement
ü Double displacement
ü Combustion
Combination reactions
In combination reactions, two or more reactants form one product. The reaction of sodium and chlorine to form sodium chloride,
![]()
and the burning of coal (carbon) to give carbon dioxide,
![]()
are examples of combination reactions.
Note that, depending on conditions or the relative amounts of the reactants, more than one product can be formed in a combination reaction. Take the burning of coal, for example. If an excess of oxygen is present, the product is carbon dioxide. But if a limited amount of oxygen is available, the product is carbon monoxide:
![]() (limited oxygen)
(limited oxygen)
Decomposition reactions
Decomposition reactions are really the opposite of combination reactions. In decomposition reactions, a single compound breaks down into two or more simpler substances (elements and/or compounds). The decomposition of water into hydrogen and oxygen gases,
![]()
and the decomposition of hydrogen peroxide to form oxygen gas and water,
![]()
are examples of decomposition reactions.
Single displacement reactions
In single displacement reactions, a more active element displaces (kicks out) another less active element from a compound. For example, if you put a piece of zinc metal into a copper(II) sulfate solution (by the way, Chapter 6 explains why copper(II) sulfate is named the way it is — in case you’re wondering), the zinc displaces the copper, as shown in this equation:
![]()
The notation (aq) indicates that the compound is dissolved in water — in an aqueous solution. Because zinc replaces copper in this case, it’s said to be more active. If you place a piece of copper in a zinc sulfate solution, nothing will happen. Table 8-1 shows the activity series of some common metals. Notice that because zinc is more active in the table, it will replace copper, just as the preceding equation shows.
Table 8-1. The Activity Series of Some Common Metals
|
Activity |
Metal |
|
Most active |
Alkali and alkaline earth metals |
|
|
Al |
|
|
Zn |
|
|
Cr |
|
|
Fe |
|
|
Ni |
|
|
Sn |
|
|
Pb |
|
|
Cu |
|
|
Ag |
|
Least Active |
Au |
Take another look at the reaction between zinc metal and copper(II) sulfate solution:
![]()
I’ve written this reaction as a molecular equation, showing all species in the neutral form. However, these reactions normally occur in an aqueous (water) solution. When the ionically-bonded CuSO4is dissolved in water, it breaks apart into ions (atoms or groups of atoms that have an electrical charge due to the loss or gain of electrons). The copper ion has a +2 charge because it lost two electrons. It’s a cation, a positively charged ion. The sulfate ion has a -2 charge because it has two extra electrons. It’s an anion, a negatively charged ion. (Check out Chapter 6 for a more complete discussion of ionic bonding.)
![]()
Equations written in this form, in which the ions are shown separately, are called ionic equations (because they show the reaction and production of ions). Notice that the sulfate ion, SO42-, hasn’t changed in the reaction. Ions that don’t change during the reaction and are found on both sides of the equation in an identical form are called spectator ions. Chemists (a lazy, lazy lot, they are) often omit the spectator ions and write the equation showing only those chemical substances that are changed during the reaction. This is called the net-ionic equation:
![]()
Double displacement reactions
In single displacement reactions, only one chemical species is displaced. In double displacement reactions, or metathesis reactions, two species (normally ions) are displaced. Most of the time, reactions of this type occur in a solution, and either an insoluble solid (precipitation reactions) or water (neutralization reactions) will be formed.
Precipitation reactions
If you mix a solution of potassium chloride and a solution of silver nitrate, a white insoluble solid is formed in the resulting solution. The formation of an insoluble solid in a solution is called precipitation. Here are the molecular, ionic, and net-ionic equations for this double-displacement reaction:
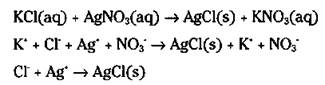
The white insoluble solid that’s formed is silver chloride. You can drop out the potassium cation and nitrate anion spectator ions, because they don’t change during the reaction and are found on both sides of the equation in an identical form. (If you’re totally confused about all those plus and minus symbols in the equations, or don’t know what a cation or an anion is, just flip to Chapter 6. It tells all you need to know about this stuff.)
In order to write these equations, you have to know something about the solubility of ionic compounds. Don’t fret. Here you go: If a compound is soluble, it will remain in its free ion form, but if it’s insoluble, it will precipitate (form a solid). Table 8-2 gives the solubilities of selected ionic compounds.
Table 8-2. Solubilities of Selected Ionic Compounds
|
Water Soluble |
Water Insoluble |
|
All chlorides, bromides, iodides |
except those of Ag+, Pb2+, Hg22+ |
|
All compound of NH4+ |
Oxides |
|
All compounds of alkali metals |
Sulfides |
|
All acetates |
most phosphates |
|
All nitrates |
most hydroxides |
|
All chlorates |
|
|
All sulfates |
except PbSO4, BaSO4 and SrSO4 |
To use Table 8-2, take the cation of one reactant and combine it with the anion of the other reactant, and vice versa (keeping the neutrality of the compounds). This allows you to predict the possible products of the reaction. Then look up the solubilities of the possible products in the table. If the compound is insoluble, it will precipitate. If it is soluble, it will remain in solution.
Neutralization reactions
The other type of double-displacement reaction is the reaction between an acid and a base. This double-displacement reaction, called a neutralization reaction, forms water. Take a look at the mixing solutions of sulfuric acid (auto battery acid, H2SO4) and sodium hydroxide (lye, NaOH). Here are the molecular, ionic, and net-ionic equations for this reaction:
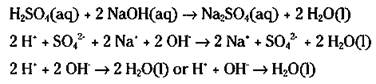
To go from the ionic equation to the net-ionic equation, the spectator ions (those that don’t really react and that appear in an unchanged form on both sides on the arrow) are dropped out. Then the coefficients in front of the reactants and products are reduced down to the lowest common denominator.
You can find more about acid-base reactions in Chapter 12.
Combustion reactions
Combustion reactions occur when a compound, usually one containing carbon, combines with the oxygen gas in the air. This process is commonly called burning. Heat is the most-useful product of most combustion reactions.
Here’s the equation that represents the burning of propane:
![]()
Propane belongs to a class of compounds called hydrocarbons, compounds composed only of carbon and hydrogen. The product of this reaction is heat. You don’t burn propane in your gas grill to add carbon dioxide to the atmosphere — you want the heat for cooking your steaks.
Combustion reactions are also a type of redox reaction.
Redox reactions
Redox reactions, or reduction-oxidation reactions, are reactions in which electrons are exchanged:
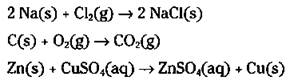
The preceding reactions are examples of other types of reactions (such as combination, combustion, and single-replacement reactions), but they’re all redox reactions. They all involve the transfer of electrons from one chemical species to another. Redox reactions are involved in combustion, rusting, photosynthesis, respiration, batteries, and more. I talk about redox reactions in some detail in Chapter 9.
Balancing Chemical Reactions
If you carry out a chemical reaction and carefully sum up the masses of all the reactants, and then compare the sum to the sum of the masses of all the products, you see that they’re the same. In fact, a law in chemistry, the Law of Conservation of Mass, states, “In an ordinary chemical reaction, matter is neither created nor destroyed.” This means that you have neither gained nor lost any atoms during the reaction. They may be combined differently, but they’re still there.
A chemical equation represents the reaction. That chemical equation is used to calculate how much of each element is needed and how much of each element will be produced. And that chemical equation needs to obey the Law of Conservation of Mass.
You need to have the same number of each kind of element on both sides of the equation. The equation should balance. In this section, I show you how to balance chemical equations.
Smelt that ammonia
My favorite reaction is called the Haber process, a method for preparing ammonia (NH3) by reacting nitrogen gas with hydrogen gas:
![]()
This equation shows you what happens in the reaction, but it doesn’t show you how much of each element you need to produce the ammonia. To find out how much of each element you need, you have to balance the equation — make sure that the number of atoms on the left side of the equation equals the number of atoms on the right.
You know the reactants and the product for this reaction, and you can’t change them. You can’t change the compounds, and you can’t change the subscripts, because that would change the compounds. So the only thing you can do to balance the equation is add coefficients, whole numbers in front of the compounds or elements in the equation. Coefficients tell you how many atoms or molecules you have.
For example, if you write 2H2O. it means you have two water molecules:
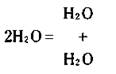
Each water molecule is composed of two hydrogen atoms and one oxygen atom. So with 2 H2O, you have a total of 4 hydrogen atoms and 2 oxygen atoms:
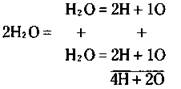
In this chapter, I show you how to balance equations by using a method called balancing by inspection, or as I call it, “fiddling with coefficients.” You take each atom in turn and balance it by adding appropriate coefficients to one side or the other.
With that in mind, take another look at the equation for preparing ammonia:
![]()
TIP. In most cases, it’s a good idea to wait until the end to balance hydrogen atoms and oxygen atoms; balance the other atoms first.
So in this example, you need to balance the nitrogen atoms first. You have 2 nitrogen atoms on the left side of the arrow (reactant side) and only 1 nitrogen atom on the right side (product side). In order to balance the nitrogen atoms, use a coefficient of 2 in front of the ammonia on the right.
![]()
Now you have 2 nitrogen atoms on the left and 2 nitrogen atoms on the right.
Next, tackle the hydrogen atoms. You have 2 hydrogen atoms on the left and 6 hydrogen atoms on the right (2 NH3 molecules, each with 3 hydrogen atoms, for a total of 6 hydrogen atoms). So put a 3 in front of the H2 on the left, giving you:
![]()
That should do it. Do a check to be sure: You have 2 nitrogen atoms on the left and 2 nitrogen atoms on the right. You have 6 hydrogen atoms on the left (3x2 = 6) and 6 hydrogen atoms on the right (2x3 = 6). The equation is balanced. You can read the equation this way: 1 nitrogen molecule reacts with 3 hydrogen molecules to yield 2 ammonia molecules.
Here’s a tidbit for you: This equation would have also balanced with coefficients of 2, 6, and 4 instead of 1,3, and 2. In fact, any multiple of 1, 3, and 2 would have balanced the equation, but chemists have agreed to always show the lowest whole-number ratio (see the discussion on empirical formulas in Chapter 7 for details).
Flick that bic
Take a look at an equation showing the burning of butane, a hydrocarbon, with excess oxygen available. (This is the reaction that takes place when you light a butane lighter.) The unbalanced reaction is
![]()
Because it’s always a good idea to wait until the end to balance hydrogen atoms and oxygen atoms, balance the carbon atoms first. You have 4 carbon atoms on the left and one carbon atom on the right, so add a coefficient of 4 in front of the carbon dioxide:
![]()
Balance the hydrogen atoms next. You have 10 hydrogen atoms on the left and 2 hydrogen atoms on the right, so use a coefficient of 5 in front of the water on the right:
![]()
Now work on balancing the oxygen atoms. You have 2 oxygen atoms on the left and a total of 13 oxygen atoms on the right [(4 x 2) + (5 x 1) = 13]. What can you multiply 2 with in order for it to equal 13? How about 6.5?
![]()
But you’re not done. You want the lowest whole-number ratio of coefficients. You’ll have to multiply the entire equation by 2 in order to generate whole numbers:
![]()
Multiply every coefficient by 2 (don’t touch the subscripts!) to get
![]()
If you check the atom count on both sides of the equation, you find that the equation is balanced, and the coefficients are in the lowest whole-number ratio.
REMEMBER. After balancing an equation, make sure that the same number of each atom is on both sides and that the coefficients are in the lowest whole-number ratio.
Most simple reactions can be balanced in this fashion. But one class of reactions is so complex that this method doesn’t work well when applied to them. They’re redox reactions. A special method is used for balancing these equations, and I show it to you in Chapter 9.
Chemical Equilibrium
My favorite reaction is the Haber process, the synthesis of ammonia from nitrogen and hydrogen gases. After balancing the reaction (see the section “Smell that ammonia,” earlier in this chapter), you end up with
![]()
Written this way, the reaction says that hydrogen and nitrogen react to form ammonia — and this keeps on happening until you use up one or both of the reactants. But this isn’t quite true. (Yep. It’s hair-splitting time.)
If this reaction occurs in a closed container (which it has to, with everything being gases), then the nitrogen and hydrogen react and ammonia is formed — but some of the ammonia soon starts to decompose to nitrogen and hydrogen, like this:
![]()
In the container, then, you actually have two exactly opposite reactions occurring — nitrogen and hydrogen combine to give ammonia, and ammonia decomposes to give nitrogen and hydrogen.
Instead of showing the two separate reactions, you can show one reaction and use a double arrow like this:
![]()
You put the nitrogen and hydrogen on the left because that’s what you initially put into the reaction container.
Now these two reactions occur at different speeds, but sooner or later, the two speeds become the same, and the relative amounts of nitrogen, hydrogen, and ammonia become constant. This is an example of a chemical equilibrium. A dynamic chemical equilibrium is established when two exactly opposite chemical reactions are occurring at the same place, at the same time, with the same rates (speed) of reaction. I call this example a dynamic chemical equilibrium, because when the reactions reach equilibrium, things don’t just stop. At any given time, you have nitrogen and hydrogen reacting to form ammonia, and ammonia decomposing to form nitrogen and hydrogen. When the system reaches equilibrium, the amounts of all chemical species become constant but not necessarily the same.
Here’s an example to help you understand what I mean by this dynamic stuff: I was raised on a farm in North Carolina, and my mother, Grace, loved small dogs. Sometimes we’d have close to a dozen dogs running around the house. When Mom opened the door to let them outside, they’d start running out. But some would change their minds after they got outside and would then start running back into the house. They’d then get caught up in the excitement of the other dogs and start running back outside again. There’d be a never-ending cycle of dogs running in and out of the house. Sometimes there’d only be two or three in the house, with the rest outside, or vice versa. The number of dogs inside and outside would be constant but not the same. And at any given point, there’d be dogs running out of the house and dogs running into the house. It was a dynamic equilibrium (and a noisy one).
Sometimes there’s a lot of product (chemical species on the right-hand side of the double arrow) when the reaction reaches equilibrium, and sometimes there’s very little. You can tell the relative amounts of reactants and products at equilibrium if you know the equilibrium constant for the reaction.
Look at a hypothetical equilibrium reaction:
![]()
The capital letters stand for the chemical species, and the small letters represent the coefficients in the balanced chemical equation. The equilibrium constant (represented as Keq) is mathematically defined as
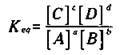
The numerator contains the product of the two chemical species on the right- hand side of the equation, with each chemical species raised to the power of its coefficient in the balanced chemical equation. The denominator is the same, but you use the chemical species on the left-hand side of the equation. (It’s not important right now, but those brackets stand for something called the molar concentration. You can find out what that is in Chapter 11.) Note that sometimes chemists use the Kc notation instead of the Keq, form.
The numerical value of the equilibrium constant gives you a clue as to the relative amounts of products and reactants.
TIP. The larger the value of the equilibrium constant (Keq), the more products are present at equilibrium. If, for example, you have a reaction that has an equilibrium constant of 0.001 at room temperature and 0.1 at 100 degrees Celsius, you can say that you will have much more product at the higher temperature.
Now I happen to know that the Keq for the Haber process (the ammonia synthesis) is 3.5 x 108 at room temperature. This large value indicates that, at equilibrium, there’s a lot of ammonia produced from the nitrogen and hydrogen, but there’s still hydrogen and nitrogen left at equilibrium. If you’re, say, an industrial chemist making ammonia, you want as much of the reactants as possible to be converted to product. You’d like the reaction to go to completion (meaning you’d like the reactants to keep creating the product until they’re all used up), but you know that it’s an equilibrium reaction, and you can’t change that. But it would be nice if you could, in some way, manipulate the system to get a little bit more product formed. There is such a way — through Le Chatelier’s Principle.
Le Chatelier’s Principle
A French chemist, Henri Le Chatelier, discovered that if you apply a change of condition (called stress) to a chemical system that’s at equilibrium, the reaction will return to equilibrium by shifting in such a way as to counteract the change (the stress). This is called Le Chatelier's Principle.
You can stress an equilibrium system in three ways:
ü Change the concentration of a reactant or product.
ü Change the temperature.
ü Change the pressure on a system that contains gases.
Now if you’re a chemist who’s looking for a way to make as much ammonia (money) as possible for a chemical company, you can use Le Chatelier’s Principle to help you along. In this section, I show you how. But first, I want to show you a quick, useful analogy. A reaction at equilibrium is like one of my favorite pieces of playground equipment, a teeter-totter. Everything is well balanced, as shown in Figure 8-3.
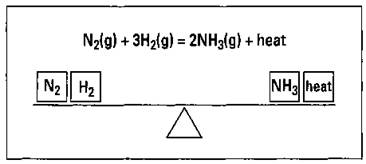
Figure 8-3: The Haber ammonia system at equilibrium.
The Haber process, the synthesis of ammonia from nitrogen and hydrogen gases, is exothermic: It gives off heat. I show that heat on the right-hand side of the teeter-totter in the figure.
Changing the concentration
Suppose that you have the ammonia system at equilibrium (see Figure 8-3, as well as the section, “Chemical Equilibrium,” earlier in this chapter), and you then put in some more nitrogen gas. Figure 8-4 shows what happens to the teeter-totter when you add more nitrogen gas.
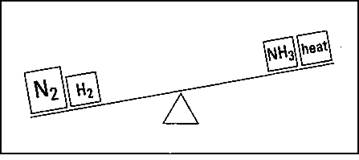
Figure 8-4: Increasing the concentration of a reactant.
In order to reestablish the balance (equilibrium), weight has to be shifted from the left to the right, using up some nitrogen and hydrogen, and forming more ammonia and heat. Figure 8-5 shows this shifting of weight.
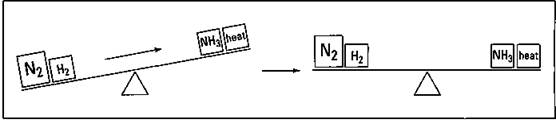
Figure 8-5: Reestablishing the equilibrium.
The equilibrium has been reestablished. There is less hydrogen and more nitrogen, ammonia, and heat than you had before you added the additional nitrogen. The same thing would happen if you had a way of removing ammonia as it was formed. The right-hand side of the teeter-totter would again be lighter, and weight would be shifted to the right in order to reestablish the equilibrium. Again, more ammonia would be formed. In general, if you add more of a reactant or product, the reaction will shift to the other side to use it up. If you remove some reactant or product, the reaction shifts to that side in order to replace it.
Changing the temperature
Suppose that you heat the reaction mixture. You know that the reaction is exothermic — heat is given off, showing up on the right-hand side of the equation. So if you heat the reaction mixture, the right side of the teeter-totter gets heavier, and weight must be shifted to the left in order to reestablish the equilibrium. This weight shift uses up ammonia and produces more nitrogen and hydrogen. And as the reaction shifts, the amount of heat also decreases, lowering the temperature of the reaction mixture. Figure 8-6 shows this shift in weight.
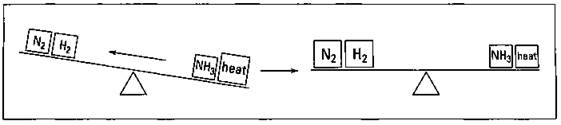
Figure 8-6: Increasing the temperature on an exothermic reaction and reestablishing the equilibrium.
That’s not what you want! You want more ammonia, not more nitrogen and hydrogen. So you have to cool the reaction mixture, using up heat, and then the equilibrium shifts to the right in order to replace it. This process helps you make more ammonia and more profit. That’s more like it.
In general, heating a reaction causes it to shift to the endothermic side. (If you have an exothermic reaction where heat is produced on the right side, then the left side is the endothermic side.) Cooling a reaction mixture causes the equilibrium to shift to the exothermic side.
Changing the pressure
Changing the pressure only affects the equilibrium if there are reactants and/or products that are gases. In the Haber process, all species are gases, so there is a pressure effect.
My teeter-totter analogy of equilibrium systems begins to break down when I explain pressure effects, so I have to take another approach. Think about the sealed container where your ammonia reaction is occurring. (The reaction has to occur in a sealed container with everything being gases.) You have nitrogen, hydrogen, and ammonia gases inside. There is pressure in the sealed container, and that pressure is due to the gas molecules hitting the inside walls of the container.
Now suppose that the system is at equilibrium, and you want to increase the pressure. You can do so by making the container smaller (with a piston type of arrangement) or by putting in an unreactive gas, such as neon. You get more collisions on the inside walls of the container, and, therefore, you have more pressure. Increasing the pressure stresses the equilibrium; in order to remove that stress and reestablish the equilibrium, the pressure must be reduced.
Take another look at the Haber reaction and see if there may be some clues as to how this may happen.
![]()
Every time the forward (left to right) reaction takes place, four molecules of gas (one nitrogen and three hydrogen) form two molecules of ammonia gas. This reaction reduces the number of molecules of gas in the container. The reverse reaction (right to left) takes two ammonia gas molecules and makes four gas molecules (nitrogen and hydrogen). This reaction increases the number of gas molecules in the container.
The equilibrium has been stressed by an increase in pressure; reducing the pressure will relieve the stress. Reducing the number of gas molecules in the container will reduce the pressure (fewer collisions on the inside walls of the container), so the forward (left to right) reaction is favored because four gas molecules are consumed and only two are formed. As a result of the forward reaction, more ammonia is produced!
In general, increasing the pressure on an equilibrium mixture causes the reaction to shift to the side containing the fewest number of gas molecules.
Reacting Fast and Reacting Stout: Chemical Kinetics
Say that you’re a chemist who wants to make as much ammonia as possible from a given amount of hydrogen and nitrogen. Manipulating the equilibrium (see the preceding section) isn’t your total solution. You want to produce as much as possible, as fast as possible. So there’s something else you must consider — the kinetics of the reaction.
Kinetics is the study of the speed of a reaction. Some reactions are fast; others are slow. Sometimes chemists want to speed the slow ones up and slow the fast ones down. There are several factors that affect the speed of a reaction:
ü Nature of the reactants
ü Particle size of the reactants
ü Concentration of the reactants
ü Pressure of gaseous reactants
ü Temperature
ü Catalysts
Mature of the reactants
In order for a reaction to occur, there must be a collision between the reactants at the reactive site of the molecule (see “How Do Reactions Occur? Collision Theory,” earlier in this chapter). The larger and more complex the reactant molecules, the less chance there is of a collision at the reactive site. Sometimes, in very complex molecules, the reactive site is totally blocked off by other parts of the molecule, so no reaction occurs. There may be a lot of collisions, but only the ones that occur at the reactive site have any chance of leading to chemical reaction.
In general, the reaction rate is slower when the reactants are large and complex molecules.
Particle size of the reactants
Reaction depends on collisions. The more surface area on which collisions can occur, the faster the reaction. You can hold a burning match to a large chunk of coal and nothing will happen. But if you take that same piece of coal, grind it up very, very fine, throw it up into the air, and strike a match, you’ll get an explosion because of the increased surface area of the coal.
Concentration of the reactants
Increasing the number of collisions speeds up the reaction rate. The more reactant molecules there are colliding, the faster the reaction will be. For example, a wood splint burns okay in air (20 percent oxygen), but it burns much faster in pure oxygen.
In most simple cases, increasing the concentration of the reactants increases the speed of the reaction. However, if the reaction is complex and has a complex mechanism (series of steps in the reaction), this may not be the case. In fact, determining the concentration effect on the rate of reaction can give you clues as to which reactant is involved in the rate-determining step of the mechanism. (This information can then be used to help figure out the reaction mechanism.) You can do this by running the reaction at several different concentrations and observing the effect on the rate of reaction. If, for example, changing the concentration of one reactant has no effect on the rate of reaction, then you know that reactant is not involved in the slowest step (the rate-determining step) in the mechanism.
Pressure of gaseous reactants
The pressure of gaseous reactants has basically the same effect as concentration. The higher the reactant pressure, the faster the reaction rate. This is due to (you guessed it!) the increased number of collisions. But if there’s a complex mechanism involved, changing the pressure may not have the expected result.
Temperature
Okay, why did mom tell you to put that turkey in the refrigerator after Thanksgiving dinner? Because it would’ve spoiled if you didn’t. And what is spoilage? It’s increased bacterial growth. So when you put the turkey in the refrigerator, the cold temperature inside the fridge slowed down the rate of bacterial growth.
Bacterial growth is simply a biochemical reaction, a chemical reaction involving living organisms. In most cases, increasing the temperature causes the reaction rate to increase. In organic chemistry, there’s a general rule that says increasing the temperature 10 degrees Celsius will cause the reaction rate to double.
But why is this true? Part of the answer is (you guessed it!) an increased number of collisions. Increasing the temperature causes the molecules to move faster, so there’s an increased chance of them colliding with each other and reacting. But this is only part of the story. Increasing the temperature also increases the average kinetic energy of the molecules. Look at Figure 8-7 for an example of how increasing the temperature affects the kinetic energy of the reactants and increases the reaction rate.
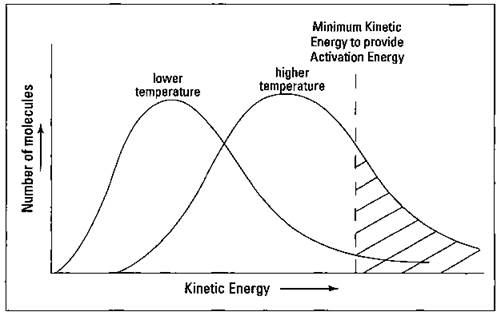
Figure 8-7: The effect of temperature on the kinetic energy of reactants.
At a given temperature, not all the molecules are moving with the same kinetic energy. A small number of molecules are moving very slow (low kinetic energy), while a few are moving very fast (high kinetic energy). A vast majority of the molecules are somewhere in between these two extremes.
In fact, temperature is a measure of the average kinetic energy of the molecules. As you can see in Figure 8-7, increasing the temperature increases the average kinetic energy of the reactants, essentially shifting the curve to the right toward higher kinetic energies. But also notice that I’ve marked the minimum amount of kinetic energy needed by the reactants to provide the activation energy (the energy required to get a reaction going) during collision. The reactants have to collide at the reactive site, but they also have to transfer enough energy to break bonds so that new bonds can be formed. If the reactants don’t have enough energy, a reaction won’t occur even if the reactants do collide at the reactive site.
Notice that at the lower temperature, very few of the reactant molecules have the minimum amount of kinetic energy needed to provide the activation energy. At the higher temperature, many more molecules possess the minimum amount of kinetic energy needed, which means a lot more collisions will be energetic enough to lead to reaction.
Increasing the temperature not only increases the number of collisions but also increases the number of collisions that are effective — that transfer enough energy to cause a reaction to take place.
Catalysts
Catalysts are substances that increase the reaction rate without themselves being changed at the end of the reaction. They increase the reaction rate by lowering the activation energy for the reaction.
Look at Figure 8-1, for example. If the activation energy hill were lower, it’d be easier for the reaction to occur and the reaction rate would be faster. You can see the same thing in Figure 8-7. If you shift to the left that dotted line representing the minimum amount of kinetic energy needed to provide the activation energy, then many more molecules will have the minimum energy needed, and the reaction will be faster.
Catalysts lower the activation energy of a reaction in one of two ways:
ü Providing a surface and orientation
ü Providing an alternative mechanism (series of steps for the reaction to go through) with a lower activation energy
Surface and orientation — heterogeneous catalysis
In the section “How Do Reactions Occur? Collision Theory,” I describe how molecules react, using this generalized example:
![]()
Reactant C must hit the reactive site on the A end of molecule AS in order to break the AS bond and form the C-A bond shown in the equation. The probability of the collision occurring in the proper orientation is pretty much driven by chance. The reactants are moving around, running into each other, and sooner or later the collision may occur at the reactive site. But what would happen if you could tie the A-B molecule down with the A end exposed? It’d be much easier and more probable for C to hit A with this scenario.
This is what a heterogeneous catalyst accomplishes: It ties one molecule to a surface while providing proper orientation to make the reaction easier. The process of heterogeneous catalysis is shown in Figure 8-8.
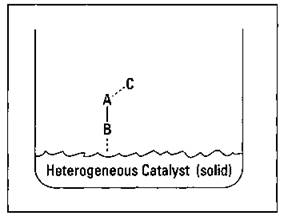
Figure 8-8: An example of heterogeneous catalysis.
The catalyst is called a heterogeneous catalyst because it’s in a different phase than the reactants. This catalyst is commonly a finely divided solid metal or metal oxide, while the reactants are gases or in solution. This heterogeneous catalyst tends to attract one part of a reactant molecule due to rather complex interactions that are not fully understood. After the reaction takes place, the forces that bound the B part of the molecule to the surface of the catalyst are no longer there. So B can drift off, and the catalyst can be ready to do it again.
Most of us sit very close to a heterogeneous catalyst every day — the catalytic converter in our automobile. It contains finely divided platinum and/or palladium metal and speeds up the reaction that causes harmful gases from gasoline (such as carbon monoxide and unburned hydrocarbons) to decompose into mostly harmless products (such as water and carbon dioxide).
Alternative mechanism — homogeneous catalysis
The second type of catalyst is a homogeneous catalyst— one that’s in the same phase as the reactants. It provides an alternative mechanism, or reaction pathway, that has a lower activation energy than the original reaction. For an example, check out the decomposition reaction of hydrogen peroxide:
![]()
This is a slow reaction, especially if it’s kept cool in a dark bottle. It may take years for that bottle of hydrogen peroxide in your medicine cabinet to decompose. But if you put a little bit of a solution containing the ferric ion in the bottle, the reaction will be much faster, even though it will be a two-step mechanism instead of a one-step mechanism:
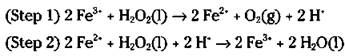
If you add the two preceding reactions together and cancel the species that are identical on both sides, you get the original, uncatalyzed reaction:
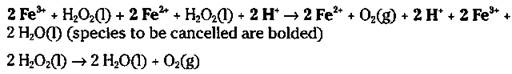
The ferric ion catalyst was changed in the first step and then changed back in the second step. This two-step catalyzed pathway has a lower activation energy and is faster.