Chemistry Essentials for Dummies
Chapter 1. Matter and Energy: Exploring the Stuff of Chemistry
In This Chapter
· Understanding the states of matter
· Differentiating between pure substances and mixtures
· Measuring matter with the metric system
· Examining the properties of chemical substances
· Discovering the different types of energy
Simply put, chemistry is a whole branch of science about matter, which is anything that has mass and occupies space. Chemistry is the study of the composition and properties of matter and the changes it undergoes.
Matter and energy are the two basic components of the universe. Scientists used to believe that these two things were separate and distinct, but now they realize that matter and energy are linked. In an atomic bomb or nuclear reactor, for instance, matter is converted into energy. (Perhaps someday science fiction will become a reality and converting the human body into energy and back in a transporter will be commonplace.)
In this chapter, you examine the different states of matter and what happens when matter goes from one state to another. I show you how to use the SI (metric) system to make matter and energy measurements, and I describe types of energy and how energy is measured.
Knowing the States of Matter and Their Changes
Matter is anything that has mass and occupies space. It can exist in one of three classic states: solid, liquid, and gas. When a substance goes from one state of matter to another, the process is called a change of state, or phase change. Some rather interesting things occur during this process, which I explain in this section.
Solids, liquids, and gases
Particles of matter behave differently depending on whether they’re part of a solid, liquid, or gas. As Figure 2-1 shows, the particles may be organized or clumped, close or spread out. In this section, you look at the solid, liquid, and gaseous states of matter.
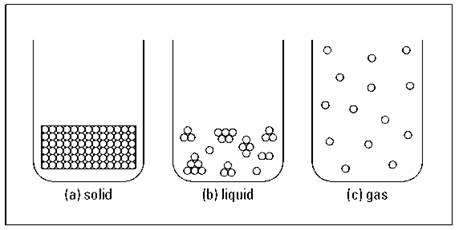
Figure 2-1: Solid, liquid, and gaseous states of matter.
Solids
At the macroscopic level, the level at which you directly observe with your senses, a solid has a definite shape and occupies a definite volume. Think of an ice cube in a glass — it’s a solid. You can easily weigh the ice cube and measure its volume.
At the microscopic level (where items are so small that people can’t directly observe them), the particles that make up the solid are very close together and aren’t moving around very much (see Figure 2-1a). That’s because in many solids, the particles are pulled into a rigid, organized structure of repeating patterns called a crystal lattice. The particles in the crystal lattice are still moving but barely — it’s more of a slight vibration. Depending on the particles, this crystal lattice may be of different shapes.
Liquids
Unlike solids, liquids have no definite shape; however, they do have a definite volume, just like solids do. The particles in liquids are much farther apart than the particles in solids, and they’re also moving around much more (see Figure 2-1b).
Even though the particles are farther apart, some particles in liquids may still be near each other, clumped together in small groups. The attractive forces among the particles aren’t as strong as they are in solids, which is why liquids don’t have a definite shape. However, these attractive forces are strong enough to keep the substance confined in one large mass — a liquid — instead of going all over the place.
Gases
A gas has no definite shape and no definite volume. In a gas, particles are much farther apart than they are in solids or liquids (see Figure 2-1c), and they’re moving relatively independent of each other. Because of the distance between the particles and the independent motion of each of them, the gas expands to fill the area that contains it (and thus it has no definite shape).
Condensing and freezing
If you cool a gaseous or liquid substance, you can watch the changes of state, or phase changes, that occur. Here are the phase changes that happen as substances lose energy:
✓ Condensation: When a substance condenses, it goes from a gas to a liquid state. Gas particles have a high amount of energy, but as they’re cooled, that energy decreases. The attractive forces now have a chance to draw the particles closer together, forming a liquid. The particles are now in clumps, as is characteristic of particles in a liquid state.
✓ Freezing: A substance freezes when it goes from a liquid to a solid. As energy is removed by cooling, the particles in a liquid start to align themselves, and a solid forms. The temperature at which this occurs is called the freezing point (fp) of the substance.
TIP. You can summarize the process of water changing from a gas to a solid in this way:
![]()
Here, the (l) stands for liquid, the (g) stands for gas, and (s) stands for solid.
Melting and boiling
As a substance heats, it can change from a solid to a liquid to a gas. For water, you represent the change like this:
![]()
This section explains melting and boiling, the changes of state that occur as a substance gains energy.
From solid to liquid
When a substance melts, it goes from a solid to a liquid state. Here’s what happens: If you start with a solid, such as ice, and take temperature readings while heating it, you find that the temperature of the solid begins to rise as the heat causes the particles to vibrate faster and faster in the crystal lattice.
After a while, some of the particles move so fast that they break free of the lattice, and the crystal lattice (which keeps a solid solid) eventually breaks apart. The solid begins to go from a solid state to a liquid state — a process called melting. The temperature at which melting occurs is called the melting point (mp) of the substance. The melting point for ice is 32°F, or 0°C.
REMEMBER. During changes of state, such as melting, the temperature remains constant — even though a liquid contains more energy than a solid. So if you watch the temperature of ice as it melts, you see that the temperature remains steady at 0°C until all the ice has melted.
TIP. The melting point (solid to a liquid) is the same as the freezing point (liquid to a solid).
From liquid to gas
The process by which a substance moves from the liquid state to the gaseous state is called boiling.
If you heat a liquid, such as a pot of cool water, the temperature of the liquid rises and the particles move faster and faster as they absorb the heat. The temperature rises until the liquid reaches the next change of state — boiling. As the particles heat up and move faster and faster, they begin to break the attractive forces between each other and move freely as a gas, such as steam, the gaseous form of water.
The temperature at which a liquid begins to boil is called the boiling point (bp). The bp depends on atmospheric pressure, but for water at sea level, it’s 212°F, or 100°C. The temperature of a boiling substance remains constant until all of it has been converted to a gas.
Skipping liquids: Sublimation
Most substances go through the logical progression from solid to liquid to gas as they’re heated (or vice versa as they’re cooled). But a few substances go directly from the solid to the gaseous state without ever becoming a liquid. Scientists call this process sublimation. Dry ice — solid carbon dioxide, written as CO2(s) — is the classic example of sublimation. You can see dry ice pieces becoming smaller as the solid begins to turn into a gas, but no liquid forms during this phase change.
The process of sublimation of dry ice is represented as
![]()
Besides dry ice, mothballs and certain solid air fresheners also go through the process of sublimation. The reverse of sublimation is deposition — going directly from a gaseous state to a solid state.