Chemistry Essentials for Dummies
Chapter 4. Nuclear Chemistry
Breaking Elements Apart with Nuclear Fission
In the 1930s, scientists discovered that some nuclear reactions can be initiated and controlled. Scientists usually accomplished this task by bombarding a large isotope with a second, smaller one — commonly a neutron. The collision caused the larger isotope to break apart into two or more elements, which is called nuclear fission. The following equation shows the nuclear fission of uranium-235:
![]()
Mass defect: Where does all that energy come from?
Nuclear fission reactions release a lot of energy. Where does the energy come from? Well, if you make very accurate measurements of the masses of all the atoms and subatomic particles you start with and all the atoms and subatomic particles you end up with, you find that some mass is “missing.” Matter disappears during the nuclear reaction. This loss of matter is called the mass defect. The missing matter is converted into energy.
You can actually calculate the amount of energy produced during a nuclear reaction with a fairly simple equation developed by Einstein: E = mc2. In this equation, E is the amount of energy produced, m is the “missing” mass, or mass defect, and c is the speed of light in a vacuum, which is a rather large number. The speed of light is squared, making that part of the equation a very large number that, even when multiplied by a small amount of mass, yields a large amount of energy.
Chain reactions and critical mass
Take a look at the equation for the fission of uranium-235 (U-235):
![]()
Notice that one neutron is used, but three are produced. These three neutrons, if they encounter other U-235 atoms, can initiate other fissions, producing even more neutrons. It’s the old domino effect — or in terms of nuclear chemistry, it’s a continuing cascade of nuclear fissions called a chain reaction. Figure 4-2 shows the chain reaction of U-235.
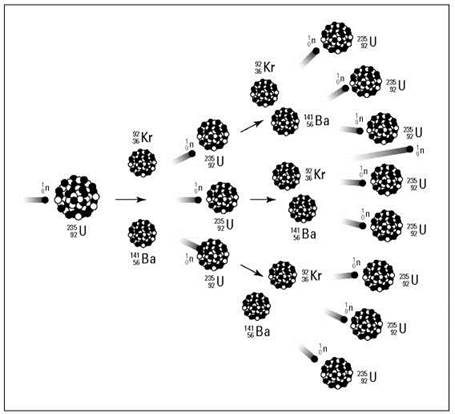
Figure 4-2: Chain reaction.
REMEMBER. A chain reaction depends on the release of more neutrons than are used during the nuclear reaction. If you were to write the equation for the nuclear fission of U-238, the more abundant isotope of uranium, you’d use one neutron and get only one back out. So you can’t have a chain reaction with U-238. But isotopes that produce an excess of neutrons in their fission support a chain reaction. This type of isotope is said to be fissionable, and only two main fissionable isotopes are used during nuclear reactions: U-235 and plutonium-239 (Pu-239).
Critical mass is the minimum amount of fissionable matter you need to support a self-sustaining chain reaction. That amount is related to those neutrons. If the sample is small, then the neutrons are likely to shoot out of the sample before hitting a U-235 nucleus. If they don’t hit a U-235 nucleus, no extra electrons and no energy are released. The reaction just fizzles. Anything less than the critical mass is called subcritical.