Chemistry Essentials for Dummies
Chapter 5. Ionic Bonding
Creating Ionic Compounds
Ionic bonding, the bonding that holds the cations and anions together, is one of the two major types of bonding in chemistry. (I describe the other type, covalent bonding, in Chapter 6.)
REMEMBER. An ionic bond occurs between a metal and a nonmetal. The metal loses electrons (to becomes a positively charged cation), and a nonmetal gains those electrons (to become a negatively charged anion). The ions have opposite charges, so they’re attracted to each other. This attraction draws them together into a compound.
In this section, you look at how ionic bonding works, and you see how to write formulas for and name ionic compounds.
Making the bond: Sodium metal + chlorine gas = sodium chloride
REMEMBER. The transfer of an electron creates ions — cations (positive charge) and anions (negative charge). Opposite charges attract each other, so the cations and anions may come together through an ionic bond. An ionic bond is a chemical bond (a strong attractive force that keeps two chemical elements together) that comes from the electrostatic attraction (attraction of opposite charges) between cations and anions. Together, the ions form a compound.
For instance, sodium, a metal, can fill its octet and achieve stability by losing an electron. Chlorine, a nonmetal, can fill its octet by gaining an electron. (See the earlier section “Gaining and losing electrons” for details on the octet rule.) If the two are in the same container, then the electron that sodium loses can be the same electron that chlorine gains. The Na+ cation attracts the Cl- anion and forms the compound NaCl, sodium chloride.
Compounds that have ionic bonds are commonly called salts. In sodium chloride — table salt — a crystal is formed in which each sodium cation is surrounded by six different chloride anions and each chloride anion is surrounded by six different sodium cations.
Different types of salts have different crystal structures. Cations and anions can have more than one unit of positive or negative charge if they lose or gain more than one electron. In this fashion, many different kinds of salts are possible.
Figuring out the formulas of ionic compounds
When an ionic compound is formed, the cation and anion attract each other, resulting in a salt. This section shows you how to write the formula of that salt.
Balancing charges: Magnesium and bromine
Suppose you want to know the formula, or composition, of a compound that results from reacting a metal and a nonmetal. You start by putting the two atoms side by side, with the metal on the left. Then you add their charges.
Figure 5-1 shows this process for magnesium and bromine. (Forget about the crisscrossing lines for now. I explain them in the upcoming section “Using the crisscross rule.”)
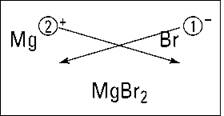
Figure 5-1: Figuring the formula of magnesium bromide.
The electron configurations for magnesium and bromine are
Magnesium (Mg): 1s22s22p63s2
Bromine (Br): 1s22s22p63s23p64s23d104p5
Magnesium, an alkaline earth metal, has two valence electrons that it loses to form a cation with a 2+ charge. The electron configuration for the magnesium cation is
Mg2+: 1s22s22p6
Bromine, a halogen, has seven valence electrons, so it gains one electron to complete its octet (eight valence electrons) and form the bromide anion with a 1- charge. The electron configuration for the bromide anion is
Br1-: 1s22s22p63s23p64s23d104p6
REMEMBER. When writing the formula of a compound, the compound must be neutral. That is, it needs to have equal numbers of positive and negative charges. So after writing the atoms, you need to balance their charges.
The magnesium ion has a 2+, so it requires two bromide anions, each with a single negative charge, to balance the two positive charges of magnesium. So the formula of the compound that results from reacting magnesium with bromine is MgBr2.
Using the crisscross rule
TIP. A quick way to determine the formula of an ionic compound is to use the crisscross rule: Take the numerical value of the metal ion’s superscript (forget about the charge symbol) and move it to the bottom right-hand side of the nonmetal’s symbol — as a subscript. Then take the numerical value of the nonmetal’s superscript and make it the subscript of the metal. (Note that if the numerical value is 1, it’s just understood and not shown.)
To see how to use this rule, look back at Figure 5-1. For magnesium and bromine, you make magnesium’s 2 a subscript of bromine and make bromine’s 1 a subscript of magnesium (but because it’s 1, you don’t show it). You get the formula MgBr2.
So what happens if you react aluminum and oxygen? Figure 5-2 shows the crisscross rule for this reaction. You get Al2O3.
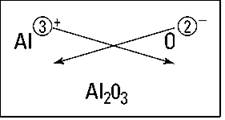
Figure 5-2: Figuring out the formula of aluminum oxide.
Compounds involving polyatomic ions work in exactly the same way. For example, here’s the compound made from the ammonium cation (NH4+) and the sulfide anion (S2-):
(NH4)2S
Notice that because you need two ammonium ions (two positive charges) to neutralize the two negative charges of the sulfide ion, you enclose the ammonium ion in parentheses and add a subscript 2.
REMEMBER. After you use the crisscross rule, reduce all the subscripts by a common factor, if possible, to get the right formula.
For example, suppose that you want to write the compound formed when calcium reacts with oxygen. Calcium, an alkaline earth metal, forms a 2+ cation, and oxygen forms a 2- anion.
So you may predict that the formula is
Mg2O2
But you need to divide each subscript by 2 to get the correct formula:
MgO
Naming ionic compounds
REMEMBER. When you name inorganic compounds, you write the name of the metal first and then the nonmetal, adding an -ide ending to the nonmetal (for compounds involving monatomic ions).
Suppose, for example, that you want to name Li2S, the compound that results from the reaction of lithium and sulfur. You first write the name of the metal, lithium, and then write the name of the nonmetal, adding an -ide ending so that sulfur becomes sulfide:
Li2S: Lithium sulfide
Ionic compounds involving polyatomic ions follow the same basic rule: Write the name of the metal first, and then simply add the name of the nonmetal. However, with polyatomic anions, it’s not necessary to add the -ide ending:
(NH4)2CO3: Ammonium carbonate
K3PO4: Potassium phosphate
Dealing with multiple oxidation states
When the metal involved is a transition metal with more than one oxidation state (see Table 5-3, earlier in the chapter), there can be more than one way to correctly name the compound, based on how you name the metal.
For example, suppose that you want to name the compound formed between the Fe3+ cation and the cyanide ion, CN-. The preferred method is to use the metal name followed in parentheses by the ionic charge written as a roman numeral: iron (III). But an older naming method, which is still sometimes used (so it’s a good idea to know it), is to use -ous and -ic endings.
REMEMBER. The ion with the lower oxidation state (lower numerical charge, ignoring the + or -) gets an -ous ending, and the ion with the higher oxidation state (higher numerical charge) gets an -ic ending. So because Fe3+ has a higher oxidation state than Fe2+, it’s called a ferric ion.
After you write the name of the metal, name the nonmetal. So the compound Fe(CN)3 can be named
Fe(CN)3: iron(III) cyanide, or ferric cyanide
Getting names from formulas and formulas from names
Sometimes figuring out the charge on an ion can be a little challenging (and fun), so take a look at how to name FeNH4(SO4)2. I show you earlier in Table 5-4 that the sulfate ion has a 2- charge, and from the formula you can see that there are two of these ions. Therefore, you have a total of four negative charges. Table 5-4 also indicates that the ammonium ion has a 1+ charge, so you can figure out the charge on the iron cation:
Ion Charge
Fe ?
NH4 1+
(SO4)2 (2-) x 2
Because you have a 4- charge for the sulfates and a 1+ for the ammonium, the iron must be a 3+ to make the compound neutral. So the iron is in the iron (III), or ferric, oxidation state. You can name the compound:
FeNH4(SO4)2: Iron (III) ammonium sulfate, or ferric ammonium sulfate
And finally, if you have the name, you can derive the formula and the charge on the ions. For example, suppose that you’re given the name cuprous oxide. You know that the cuprous ion is Cu+ and the oxide ion is O2-. Applying the crisscross rule (from the earlier section “Using the crisscross rule”), you get the following formula:
Cuprous oxide: Cu2O