Chemistry Essentials for Dummies
Chapter 6. Covalent Bonding
Electronegativities: Which Atoms Have More Pull?
Atoms may share electrons through covalent bonds, but that doesn’t mean they share equally. When the two atoms involved in a bond aren’t the same, the two positively charged nuclei have different attractive forces; they “pull” on the electron pair to different degrees. The end result is that the electron pair is shifted toward one atom. But the question is, “Which atom does the electron pair shift toward?” Electronegativities provide the answer.
REMEMBER. Electronegativity is the strength an atom has to attract a bonding pair of electrons to itself. The larger the electronegativity value, the greater the atom’s strength to attract a bonding pair of electrons.
Figure 6-10 shows the electronegativity values of the various elements below each element symbol on the periodic table. Notice that with a few exceptions, the electronegativities increase from left to right in a period and decrease from top to bottom in a family.
Predicting the type of bond
Electronegativities are useful because they give information about what will happen to the bonding pair of electrons when two atoms bond.
A bond in which the electron pair is equally shared is called a nonpolar covalent bond. You have a nonpolar covalent bond anytime the two atoms involved in the bond are the same or anytime the difference in the electronegativities of the atoms involved in the bond is very small. For example, consider the Cl2 molecule. The table in Figure 6-10 shows that chlorine has an electronegativity value of 3.0. Each chlorine atom attracts the bonding electrons with a force of 3.0. Because there’s an equal attraction, the bonding electron pair is shared equally between the two chlorine atoms and is located halfway between the two atoms.
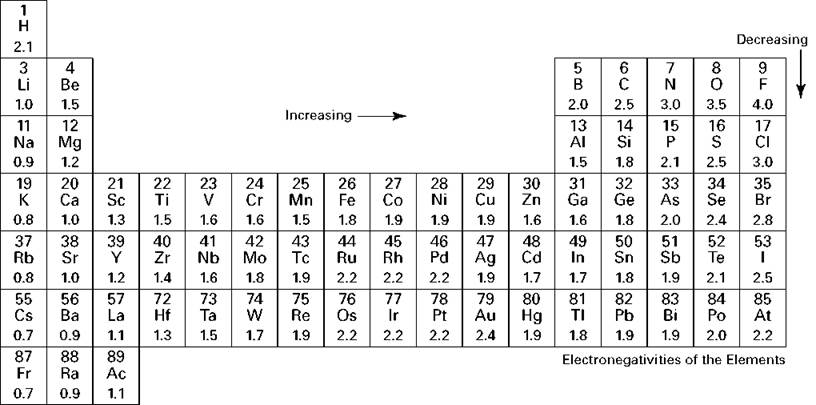
Figure 6-10: Electronegativities of the elements.
REMEMBER. A bond in which the electron pair is shifted toward one atom is called a polar covalent bond. The atom that more strongly attracts the bonding electron pair is slightly more negative, and the other atom is slightly more positive. The larger the difference in the electronegativities, the more negative and positive the atoms become. Consider hydrogen chloride (HCl). Hydrogen has an electronegativity of 2.1, and chlorine has an electronegativity of 3.0. Because chlorine has a larger electronegativity value, the electron pair that’s bonding HCl together shifts toward the chlorine atom.
If the two atoms have extremely different electronegativities, the atoms will probably form ionic, not covalent bonds. For instance, sodium chloride (NaCl) is ionically bonded. An electron has transferred from sodium to chlorine. Sodium has an electronegativity of 1.0, and chlorine has an electronegativity of 3.0. That’s an electronegativity difference of 2.0 (3.0 - 1.0), making the bond between the two atoms very, very polar.
TIP. The electronegativity difference provides another way of predicting which kind of bond will form between two elements. Here are some guidelines on whether a bond will be polar or nonpolar, covalent or ionic:
|
Electronegativity Difference |
Type of Bond Formed |
|
0.0 to 0.2 |
Nonpolar covalent |
|
0.3 to 1.4 |
Polar covalent |
|
> 1.5 |
Ionic |
The presence of a polar covalent bond in a molecule can have some pretty dramatic effects on the properties of a molecule, as you see in the next section.
Polar covalent bonding: Creating partial charges
REMEMBER. If the two atoms involved in the covalent bond are not the same, the bonding pair of electrons is pulled toward one atom, with that atom taking on a slight (partial) negative charge and the other atom taking on a partial positive charge.
In most cases, the molecule has a positive end and a negative end, called a dipole (think of a magnet). Figure 6-11 shows a couple of examples of molecules in which dipoles have formed. (The δ symbol by the charges is the lowercase Greek letter delta, and it refers to a partial charge.)
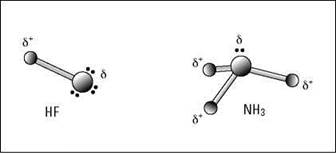
Figure 6-11: Polar covalent bonding in HF and NH3.
In hydrogen fluoride (HF), the bonding electron pair is pulled much closer to the fluorine atom than to the hydrogen atom, so the fluorine end becomes partially negatively charged and the hydrogen end becomes partially positively charged. The same thing takes place in ammonia (NH3): The nitrogen has a greater electronegativity than hydrogen, so the bonding pairs of electrons are more attracted to it than to the hydrogen atoms. The nitrogen atom takes on a partial negative charge, and each hydrogen atom takes on a partial positive charge.
REMEMBER. The presence of a polar covalent bond explains why some substances act the way they do in a chemical reaction: Because a polar molecule has a positive end and a negative end, it can attract the part of another molecule that has the opposite charge. (See the next section for details.)
In addition, a polar covalent molecule can act as a weak electrolyte because a polar covalent bond allows the substance to act as a conductor. So if a chemist wants a material to act as a good insulator (a device used to separate conductors), he or she looks for a material with as weak of a polar covalent bond as possible.
Attracting other molecules: Intermodular forces
A polar molecule is a dipole — with one end having a partial negative charge and the other end having a partial positive charge — so it acts like a magnet. These charged ends can attract other molecules. For instance, the partially negatively charged oxygen atom of one water molecule can attract the partially positively charged hydrogen atom of another water molecule. This attraction between the molecules occurs frequently and is a type of intermodular force (force between different molecules).
REMEMBER. Intermolecular forces can be of three different types:
✓ London force (dispersion force): This very weak type of attraction generally occurs between nonpolar covalent molecules, such as nitrogen (N2), hydrogen (H2), or methane (CH4). It results from the ebb and flow of the electron orbitals, giving a very weak and very brief charge separation around the bond.
✓ Dipole-dipole interaction: This intermolecular force occurs when the positive end of one dipole molecule is attracted to the negative end of another dipole molecule. It’s much stronger than a London force, but it’s still pretty weak.
✓ Hydrogen bond: The third type of interaction is really just an extremely strong dipole-dipole interaction that occurs when a hydrogen atom is bonded to one of three extremely electronegative elements: O, N, or F. These three elements have a very strong attraction for the bonding pair of electrons, so the atoms involved in the bond take on a large amount of partial charge. This bond turns out to be highly polar — and the higher the polarity, the more effective the bond.
When the O, N, or F on one molecule attracts the hydrogen of another molecule, the dipole-dipole interaction is very strong. This strong interaction (only about 5 percent of the strength of an ordinary covalent bond but still very strong for an intermolecular force) is called a hydrogen bond. The hydrogen bond is the type of interaction that’s present in water.